Traveling waves in actin dynamics and cell motility - PubMed (original) (raw)
Review
Traveling waves in actin dynamics and cell motility
Jun Allard et al. Curr Opin Cell Biol. 2013 Feb.
Abstract
Much of current understanding of cell motility arose from studying steady treadmilling of actin arrays. Recently, there have been a growing number of observations of a more complex, non-steady, actin behavior, including self-organized waves. It is becoming clear that these waves result from activation and inhibition feedbacks in actin dynamics acting on different scales, but the exact molecular nature of these feedbacks and the respective roles of biomechanics and biochemistry are still unclear. Here, we review recent advances achieved in experimental and theoretical studies of actin waves and discuss mechanisms and physiological significance of wavy protrusions.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
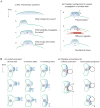
Figure 1. Experimental observations of actin traveling waves
(A) Waves of YFP-Hem1 on the ventral surface of neutrophils (reproduced from [9] under the CCA License). Time is indicated by color as the wave spreads outward. (B) Rearward waves of alpha-actinin in fibroblasts shown in micrograph (left) and kymograph (right) (reproduced from [14] with permission). Scale bars 2 microns, 30 sec. (C) Wave of protrusion across the keratocyte’s leading edge (provided by E. Barnhart).
Figure 2. Major questions
(A) Mechanisms of waving: (i) T-waves arising from excitability require an initial trigger, typically above a threshold, to initiate a wave (a–b). Once one subcellular region is excited, neighboring regions must be coupled for the wave to propagate (b–c). Many cells exhibit transient wave pulses, after which the region returns to its initial state (c–d). This return is posited to arise because of the depletion of a promoter or replenishment of an inhibitor. (ii) Three possible spatial couplings. (a) Polymerization of actin with a lateral component could transports the excited state. (b) Diffusion of a regulator. (c) Transmission of stress to neighboring regions. The stress could be mediated by the membrane or actin(-myosin) gel. (B) Possible functional roles of waving. (i) Migration in the face of limited resources. Unable to protrude uniformly along the entire leading edge, cells may focus their protrusive machinery to a limited region. If this region is stationary (a), protrusion may result in fingering and translocation of the cell body will not occur. (An alternative is narrowing of the migrating cell.) If the protruding region moves randomly (b), cell coherence could be jeopardized. A sequence of traveling waves (c) results in smooth translocation of the cell body, without affecting cell width. (ii) Avoidance of obstacles. A uniformly protruding leading edge could become stuck upon encountering an obstacle (black circle) if the stalled region (red) has no effective means of communicating with nearby regions of the edge. Waves of protrusion may circumvent this problem since the direction of cell migration is defined locally.
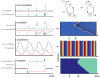
Box figure
Biochemical networks (top right) comprised of fast positive and slow negative feedbacks can exhibit qualitatively different behavior (time series, left) depending on parameters, on several timescales. Blue and red curves show behavior of molecular species A and B respectively. Steady state values of A marked by ss. Green arrows are external stimuli which instantaneously increases A and, if above a threshold (dashed lines), may relax to the only steady state, induce transient excitations in excitable systems, switch to a new steady state in bistable systems, or start fluctuating in oscillatory systems. In a region of space where each location exhibits the local dynamics shown on the left, as well as a spatial coupling to neighboring locations, spatiotemporal patterns of pulse wave, spatially uniform periodic oscillations, or wave of invasion emerge (kymographs, right).
Similar articles
- Actin Waves: Origin of Cell Polarization and Migration?
Inagaki N, Katsuno H. Inagaki N, et al. Trends Cell Biol. 2017 Jul;27(7):515-526. doi: 10.1016/j.tcb.2017.02.003. Epub 2017 Mar 7. Trends Cell Biol. 2017. PMID: 28283221 Review. - Dendritic actin filament nucleation causes traveling waves and patches.
Carlsson AE. Carlsson AE. Phys Rev Lett. 2010 Jun 4;104(22):228102. doi: 10.1103/PhysRevLett.104.228102. Epub 2010 Jun 1. Phys Rev Lett. 2010. PMID: 20867207 Free PMC article. - Control of actin filament treadmilling in cell motility.
Bugyi B, Carlier MF. Bugyi B, et al. Annu Rev Biophys. 2010;39:449-70. doi: 10.1146/annurev-biophys-051309-103849. Annu Rev Biophys. 2010. PMID: 20192778 Review. - Cell motility resulting from spontaneous polymerization waves.
Doubrovinski K, Kruse K. Doubrovinski K, et al. Phys Rev Lett. 2011 Dec 16;107(25):258103. doi: 10.1103/PhysRevLett.107.258103. Epub 2011 Dec 16. Phys Rev Lett. 2011. PMID: 22243118 - Global treadmilling coordinates actin turnover and controls the size of actin networks.
Carlier MF, Shekhar S. Carlier MF, et al. Nat Rev Mol Cell Biol. 2017 Jun;18(6):389-401. doi: 10.1038/nrm.2016.172. Epub 2017 Mar 1. Nat Rev Mol Cell Biol. 2017. PMID: 28248322 Review.
Cited by
- Excitable Rho dynamics control cell shape and motility by sequentially activating ERM proteins and actomyosin contractility.
Marshall-Burghardt S, Migueles-Ramírez RA, Lin Q, El Baba N, Saada R, Umar M, Mavalwala K, Hayer A. Marshall-Burghardt S, et al. Sci Adv. 2024 Sep 6;10(36):eadn6858. doi: 10.1126/sciadv.adn6858. Epub 2024 Sep 6. Sci Adv. 2024. PMID: 39241071 Free PMC article. - Systemic cellular migration: The forces driving the directed locomotion movement of cells.
De la Fuente IM, Carrasco-Pujante J, Camino-Pontes B, Fedetz M, Bringas C, Pérez-Samartín A, Pérez-Yarza G, López JI, Malaina I, Cortes JM. De la Fuente IM, et al. PNAS Nexus. 2024 Apr 20;3(5):pgae171. doi: 10.1093/pnasnexus/pgae171. eCollection 2024 May. PNAS Nexus. 2024. PMID: 38706727 Free PMC article. - Mesenchymal cell migration on one-dimensional micropatterns.
Heyn JCJ, Rädler JO, Falcke M. Heyn JCJ, et al. Front Cell Dev Biol. 2024 Apr 16;12:1352279. doi: 10.3389/fcell.2024.1352279. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38694822 Free PMC article. Review. - Change in RhoGAP and RhoGEF availability drives transitions in cortical patterning and excitability in Drosophila.
Jackson JA, Denk-Lobnig M, Kitzinger KA, Martin AC. Jackson JA, et al. Curr Biol. 2024 May 20;34(10):2132-2146.e5. doi: 10.1016/j.cub.2024.04.021. Epub 2024 Apr 29. Curr Biol. 2024. PMID: 38688282 - Patterning of the cell cortex by Rho GTPases.
Bement WM, Goryachev AB, Miller AL, von Dassow G. Bement WM, et al. Nat Rev Mol Cell Biol. 2024 Apr;25(4):290-308. doi: 10.1038/s41580-023-00682-z. Epub 2024 Jan 3. Nat Rev Mol Cell Biol. 2024. PMID: 38172611 Review.
References
- Bray D. Cell Movements. Garland Science; 2001.
- Sheetz MP, Felsenfeld D, Galbraith CG, Choquet D. Cell migration as a five-step cycle. Biochem Soc Symp. 1999;65:233–243. - PubMed
- Borisy GG, Svitkina TM. Actin machinery: pushing the envelope. Curr Opin Cell Biol. 2000;12:104–112. - PubMed
- Carlsson AE. Dendritic Actin Filament Nucleation Causes Traveling Waves and Patches. Phys Rev Lett. 2010;104:228102. Anders Carlsson uses his pioneering stochastic simulation method to demonstrate computationally that actin growth via branching at a cell membrane containing nucleation-promoting factors transitions from patches of actin to waves at high actin concentration. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources