Dynein mediates the localization and activation of mTOR in normal and human cytomegalovirus-infected cells - PubMed (original) (raw)
Dynein mediates the localization and activation of mTOR in normal and human cytomegalovirus-infected cells
Amy J Clippinger et al. Genes Dev. 2012.
Abstract
Activation of stress signaling pathways normally leads to inhibition of the mammalian target of rapamycin complex 1 (mTORC1); however, human cytomegalovirus (HCMV) infection maintains mTORC1 activity in the presence of numerous types of stress. We previously demonstrated that HCMV infection maintains mTORC1 activity during amino acid deprivation through a Ras-related GTP-binding (Rag) protein-independent mechanism. This depends on the colocalization of mTOR and its activator, Rheb (Ras homology enriched in brain)-GTP, to a perinuclear position that corresponds to the viral cytoplasmic assembly compartment (AC). The data presented here show that the HCMV-induced, amino acid depletion-resistant perinuclear localization and activation of mTORC1 occurs as early as 8 h post-infection, prior to AC formation. We show that the molecular motor dynein is required for perinuclear localization of mTORC1 in both uninfected and HCMV-infected cells. Association between dynein and mTOR is shown by coimmunoprecipitation, and inhibition of dynein function using RNAi or the small molecule inhibitor ciliobrevin A inhibits mTORC1 activity in both uninfected and HCMV-infected cells. The data suggest that mTORC1 activation requires dynein-dependent transport to a position in the cell where it can be activated. Thus, the HCMV commandeers a cellular dynein-dependent mTORC1 activation mechanism to maintain stress-resistant mTORC1 activity during infection and to form the AC.
Figures
Figure 1.
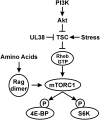
mTORC1 regulation. Activation of the PI3K (phosphatidylinositol 3-kinase)–Akt–TSC–mTORC1 pathway is critical for the phosphorylation of 4E-BP1 and S6K and the maintenance of translation. Numerous types of stresses inhibit this pathway, halting translation. HCMV has multiple mechanisms to circumvent the effects of stress signaling to maintain translation under stress conditions (see the text for details). The mechanisms for amino acid control of mTORC1 are also shown.
Figure 2.
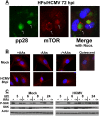
The perinuclear localization and activity of mTORC1 are maintained following amino acid deprivation as early as 8 h after HCMV infection. (A) HCMV-infected HFs (72 hpi) were stained for pp28 or mTOR followed by immunofluorescence microscopy. This shows the characteristics of perinuclear viral cytoplasmic ACs and the enlarged kidney-shaped nuclei around them. (Green) pp28; (red) mTOR; (blue) nuclei; (arrows) ACs. (B) Mock- or HCMV-infected HFs (8 hpi) were maintained in amino acid-containing medium (+AAs), amino acid-starved for 50 min prior to cell fixation (−AAs), or amino acid-starved for 50 min followed by the readdition of amino acids for 30 min prior to cell fixation (−/+AAs). Cells were then stained for mTOR followed by immunofluorescence microscopy. (Red) mTOR; (blue) nuclei. (Top right panel) An example of mTOR staining (red) in quiescent, mock-infected HFs is shown to contrast the more diffuse mTOR localization pattern of quiescent cells with its more concentrated localization in actively growing HFs (+AAs). (C) The phosphorylation of S6K was examined by Western analysis at 3, 6, 8, and 24 h after mock or HCMV infection. HFs were maintained in amino acid-containing medium (+), amino acid-starved for 50 min prior to cell collection (−), or amino acid-starved for 50 min followed by the readdition of amino acids for 30 min prior to cell collection (−/+).
Figure 3.
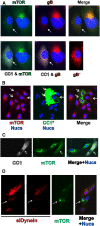
Dynein function is required for perinuclear localization of mTOR in mock- and HCMV-infected cells. (A) mTOR (green) and gB (red) staining in HCMV-infected HFs (72 hpi); the cell on the left (indicated by the arrow) is also expressing CC1 (white). (B) mTOR (red) staining in mock-infected U373-MG cells; three cells, indicated by arrows, are expressing CC1 (green). (C) mTOR (green) staining in mock-infected HFs; the cell indicated by the arrow is expressing CC1 (white). (D) mTOR (green) staining in HCMV-infected U373-MG cells; the red cells were also electroporated with siRNA that specifically targets dynein heavy chain and siGLO, a fluorescently labeled, nonspecific siRNA that marks the transfected cells. The cell indicated by the arrow has taken up no siRNA.
Figure 4.
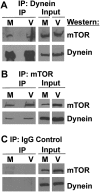
Coimmunoprecipitation of dynein and mTOR. Mock- or HCMV-infected HF lysates (72 hpi) were immunoprecipitated with mouse anti-dynein intermediate chain (A), goat anti-mTOR (B), or a nonspecific goat IgG control (C); a mouse IgG control gave the same result as the goat IgG control (not shown). Western analysis was then performed for mTOR and dynein heavy chain. (M) Mock-infected; (V) HCMV-infected.
Figure 5.
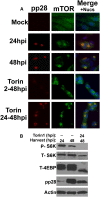
mTOR activity is not necessary for its perinuclear localization in an established infection. (A) Immunofluorescence analysis of mock- or HCMV-infected HFs at 24 or 48 hpi. Where applicable, HCMV-infected cells were treated with the mTOR kinase inhibitor Torin1 from 2 to 48 hpi (Torin 2-48hpi) or from 24 to 48 hpi (Torin 24-48hpi). (Red) pp28; (green) mTOR; (blue) nuclei. (B) Western analysis of phosphor S6K (P-S6K), total S6K (T-S6K), and total 4E-BP1 (T-4E-BP1) shows the effectiveness of Torin1 inhibition of mTORC1. Note also the expected decrease in pp28 detected in HCMV-infected cells treated with Torin1 from 2 to 48 and 24 to 48 hpi compared with untreated cells at 48 hpi.
Figure 6.
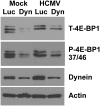
Dynein depletion inhibits mTORC1 activity. HFs were infected with lentiviral vectors expressing shRNA specific for luciferase (Luc), a control, or dynein heavy chain (Dyn) for 24 h followed by mock or HCMV infection. Seventy-two hours post HCMV infection, cells were lysed and proteins were separated by SDS-PAGE followed by Western analysis for total 4E-BP (T-4E-BP1), phospho-4E-BP (P-4-EBP1 37/46), dynein, and actin.
Figure 7.
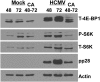
Inhibition of dynein function using CA causes loss of mTORC1 activity. HFs were mock- or HCMV-infected for 48 or 72 h and then extracted, or treated with the dynein inhibitor CA (60 μg/mL) between 48 and 72 h after mock or HCMV infection and then harvested. Western analysis was performed for total 4E-BP1 (T-4E-BP1), phospho-S6K (P-S6K), total p70 S6K (T-S6K), pp28, and actin.
Figure 8.
Inhibition of dynein function using CA affects AC formation. HFs were mock- or HCMV-infected for 48 or 72 h and then fixed, or treated with the dynein inhibitor CA (60 μg/mL) between 48 and 72 h and then fixed. Immunofluorescence analysis for pp28 (green) and mTOR (red) is shown; nuclei were stained with 4′6′-diamidino-2-phenylindole (DAPI) (blue). The HCMV used encodes GFP; thus, infected cells are indicated in the GFP panels (green). This allows uninfected cells (arrows) to be seen as internal, uninfected controls.
Figure 9.
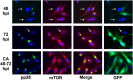
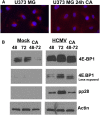
Inhibition of dynein function using CA affects the activity and perinuclear localization of mTOR in uninfected U373-MG cells. (A) Monolayers of U373-MG cells were treated with CA or the solvent control for 24 h and then stained for mTOR (red) and the nuclei (blue). (B) Monolayers of U373-MG cells were mock-infected or HCMV-infected for 48 and 72 h or treated with CA between 48 and 72 h after mock or HCMV infection. Cells were extracted for Western analysis of 4E-BP1, pp28, and actin. A lighter exposure of the 4E-BP1 analysis is provided in order to see the details in the HCMV-infected cell samples.
Similar articles
- Tuberous Sclerosis Complex Protein 2-Independent Activation of mTORC1 by Human Cytomegalovirus pUL38.
Bai Y, Xuan B, Liu H, Zhong J, Yu D, Qian Z. Bai Y, et al. J Virol. 2015 Aug;89(15):7625-35. doi: 10.1128/JVI.01027-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972538 Free PMC article. - Human cytomegalovirus infection maintains mTOR activity and its perinuclear localization during amino acid deprivation.
Clippinger AJ, Maguire TG, Alwine JC. Clippinger AJ, et al. J Virol. 2011 Sep;85(18):9369-76. doi: 10.1128/JVI.05102-11. Epub 2011 Jul 6. J Virol. 2011. PMID: 21734039 Free PMC article. - Amino acid regulation of TOR complex 1.
Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Avruch J, et al. Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E592-602. doi: 10.1152/ajpendo.90645.2008. Epub 2008 Sep 2. Am J Physiol Endocrinol Metab. 2009. PMID: 18765678 Free PMC article. Review. - The changing role of mTOR kinase in the maintenance of protein synthesis during human cytomegalovirus infection.
Clippinger AJ, Maguire TG, Alwine JC. Clippinger AJ, et al. J Virol. 2011 Apr;85(8):3930-9. doi: 10.1128/JVI.01913-10. Epub 2011 Feb 9. J Virol. 2011. PMID: 21307192 Free PMC article. - Key mediators of intracellular amino acids signaling to mTORC1 activation.
Duan Y, Li F, Tan K, Liu H, Li Y, Liu Y, Kong X, Tang Y, Wu G, Yin Y. Duan Y, et al. Amino Acids. 2015 May;47(5):857-67. doi: 10.1007/s00726-015-1937-x. Epub 2015 Feb 21. Amino Acids. 2015. PMID: 25701492 Review.
Cited by
- Tuberous Sclerosis Complex Protein 2-Independent Activation of mTORC1 by Human Cytomegalovirus pUL38.
Bai Y, Xuan B, Liu H, Zhong J, Yu D, Qian Z. Bai Y, et al. J Virol. 2015 Aug;89(15):7625-35. doi: 10.1128/JVI.01027-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972538 Free PMC article. - Virus-induced FoxO factor facilitates replication of human cytomegalovirus.
Sleman S. Sleman S. Arch Virol. 2022 Jan;167(1):109-121. doi: 10.1007/s00705-021-05279-5. Epub 2021 Nov 9. Arch Virol. 2022. PMID: 34751815 - Targeting the lysosome: Mechanisms and treatments for nonalcoholic fatty liver disease.
Pu J. Pu J. J Cell Biochem. 2022 Oct;123(10):1624-1633. doi: 10.1002/jcb.30274. Epub 2022 May 23. J Cell Biochem. 2022. PMID: 35605052 Free PMC article. Review. - Cell size sensing-a one-dimensional solution for a three-dimensional problem?
Rishal I, Fainzilber M. Rishal I, et al. BMC Biol. 2019 Apr 29;17(1):36. doi: 10.1186/s12915-019-0655-3. BMC Biol. 2019. PMID: 31035993 Free PMC article. - Host translation at the nexus of infection and immunity.
Mohr I, Sonenberg N. Mohr I, et al. Cell Host Microbe. 2012 Oct 18;12(4):470-83. doi: 10.1016/j.chom.2012.09.006. Cell Host Microbe. 2012. PMID: 23084916 Free PMC article. Review.
References
- Alwine JC. 2008. Modulation of host cell stress responses by human cytomegalovirus. In Current topics in microbiology and immunology, human cytomegalovirus (ed. TE Shenk and MF Stinski), pp. 263–279. Springer, New York.
- Buchkovich NJ. 2010. “The role of the unfolded protein response regulator BiP in HCMV virion assembly and egress.” PhD thesis, University of Pennsylvania, Philadelphia, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous