Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells - PubMed (original) (raw)
. 2012 Nov;139(21):4029-39.
doi: 10.1242/dev.082776. Epub 2012 Sep 19.
Affiliations
- PMID: 22992958
- PMCID: PMC3472587
- DOI: 10.1242/dev.082776
Draper acts through the JNK pathway to control synchronous engulfment of dying germline cells by follicular epithelial cells
Jon Iker Etchegaray et al. Development. 2012 Nov.
Abstract
The efficient removal of dead cells is an important process in animal development and homeostasis. Cell corpses are often engulfed by professional phagocytes such as macrophages. However, in some tissues with limited accessibility to circulating cells, engulfment is carried out by neighboring non-professional phagocytes such as epithelial cells. Here, we investigate the mechanism of corpse clearance in the Drosophila melanogaster ovary, a tissue that is closed to circulating cells. In degenerating egg chambers, dying germline cells are engulfed by the surrounding somatic follicular epithelium by unknown mechanisms. We show that the JNK pathway is activated and required in engulfing follicle cells. We find that the receptor Draper is also required in engulfing follicle cells, and activates the JNK pathway. Overexpression of Draper or the JNK pathway in follicle cells is sufficient to induce death of the underlying germline, suggesting that there is coordination between the germline and follicular epithelium to promote germline cell death. Furthermore, activation of JNK bypasses the need for Draper in engulfment. The induction of JNK and Draper in follicle cells occurs independently of caspase activity in the germline, indicating that at least two pathways are necessary to coordinate germline cell death with engulfment by the somatic epithelium.
Figures
Fig. 1.
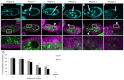
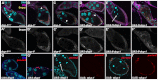
Progression of cell death and engulfment. Egg chambers from starved flies were labeled with DAPI to label DNA (cyan, top panels) and α-Dlg (magenta). Egg chambers express a germline-specific GFP gene trap (G89, green). Bottom panels are enlargements of boxed regions in middle panels. The state of chromatin condensation and fragmentation is used to characterize the different ‘phases’ of egg chamber degeneration. Egg chambers in Figs 1, 2, 3, 4, 5 and 6 are from stage 8 to early stage 9 of oogenesis. (A) Healthy (phase 0) egg chamber shows dispersed chromatin in nurse cell (NC) nuclei, and follicle cell (FC) nuclei surround the egg chamber. (B-F) Progression of cell death. NC nuclei become highly condensed and fragmented (arrows). Middle and bottom panels show that FC membranes enlarge and engulf germline GFP (arrowheads). (B) Phase 1: NC chromatin is disordered. (C) Phase 2: NC chromatin is condensed but individual nuclear regions are still apparent. (D) Phase 3: NC chromatin becomes highly condensed into individual balls. (E) Phase 4: NC chromatin is fragmented and widely dispersed. (F) Phase 5: few NC nuclear fragments remain. Scale bar: 50 μm in top and middle rows. (G) Quantification of engulfment from control G71/+ and GR1-GAL4 G89/TM6B flies shows decrease in germline GFP and area as death progresses. Data are mean±s.e.m.
Fig. 2.
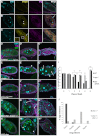
Draper is required in follicle cells for proper engulfment of nurse cells. (A-C) Wild-type (w1118) egg chambers labeled with DAPI (cyan), α-Drpr (yellow) and α-Dlg (magenta) (from starved flies). (A) Healthy egg chamber. (B) Phase 3 dying egg chamber. (C) Phase 5 egg chamber. Drpr staining intensity increases in the follicle cells (FCs) (arrowheads in B) as engulfment proceeds in dying egg chambers. Arrow in B merge shows internalization of a nurse cell (NC) nuclear fragment; arrow and inset in C indicate Drpr puncta within FCs. (D-H″) Egg chambers from starved flies expressing germline specific GFP (G71, green) stained with DAPI (DNA, cyan) and α-Dlg (magenta). Egg chambers are phases 0, 3 and 5 (left to right). (D-D″) Control G71/+ egg chambers show normal death and engulfment. (E-E″) _drpr_Δ5 flies show normal healthy egg chambers (E) but are defective in engulfment (E′) and show premature FC death (arrowheads) and lingering germline debris (arrows) (E″). (F-F″) Expression of drpr dsRNA in the FCs with GRI-GAL4 shows the same phenotype. (G-G″) Expression of drpr+ in the FCs of _drpr_Δ5 egg chambers rescues engulfment defects. FCs enlarge and take up NC debris (arrows). (H-H″) Overexpression of drpr in the FCs of otherwise wild-type egg chambers induces NC death (flies not starved). FCs first thin out (arrowheads) (H′) but engulfment eventually begins and proceeds normally (H″). Scale bars: 50 μm. (I) Quantification of unengulfed germline (wild type data from Fig. 1G). Degree and pattern of chromatin condensation were used as the primary criteria for assigning phases of death in mutant egg chambers. For phase 5 egg chambers, a reduced number of NC nuclear fragments and/or over 50% pyknotic FC nuclei were additional criteria. Phase 5 drpr egg chambers show over 100% unengulfed germline because of FC death. *P<0.05, **P<0.005, ***P<0.001. Data are mean±s.e.m. (J) Percentages of phase 3-5 egg chambers that show no engulfment, partial engulfment (less than wild type), complete engulfment (similar to wild type) and hyper-engulfment (engulfment before NC chromatin condensation) for _drpr_Δ5 (_n_=34) and UAS-drpr; _drpr_Δ5 GR1-GAL4 (_n_=192).
Fig. 3.
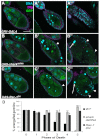
Shark and Rac1 are required in follicle cells for proper engulfment. Egg chambers from starved flies express G71 GFP in the germline, stained with α-Dlg (magenta) and DAPI (cyan). (A-A″) GRI-GAL4 alone shows normal progression of mid-oogenesis PCD. (B-B″) UAS-sharkdsRNA/+; GRI-GAL4/+ shows defective clearance of nurse cells (arrow). Follicle cell (FC) membranes do not enlarge and GFP is largely absent in the FCs (arrowhead in B′). FCs are pyknotic (arrowheads in B″), as in _drpr_Δ5 mutants. (C-C″) UAS-Rac1DN/+; GRI-GAL4/+ shows same phenotype. Scale bar: 50 μm. (D) Quantification of the unengulfed area. Data are mean±s.e.m. *P<0.05, ***P<0.001.
Fig. 4.
The JNK pathway is activated and required in follicle cells during engulfment. (A-D) Healthy and progressively dying egg chambers from starved flies are stained with DAPI (cyan), α-Drpr (yellow) and α-β-gal (red). Egg chambers carry a lacZ enhancer trap in puc. (A) Healthy egg chambers express minimal Drpr and no puc-lacZ. (B) Phase 1 dying egg chambers begin to express Drpr and puc-lacZ in the follicle cells (FCs) (arrowheads). (C,D) Drpr and puc-lacZ are expressed robustly in actively engulfing FCs in phase 4-5 dying egg chambers. (E-G‴) Healthy and progressively dying egg chambers from starved JNK pathway mutants stained with DAPI (cyan) and α-Dlg (magenta). Arrowheads indicate the pyknotic nuclei of dying FCs or FCs that have failed to enlarge. (E-E‴) Egg chambers from UAS-bskDN/+; GR1-GAL4 G89/+ flies express bskDN in the FCs and germline GFP (green). Healthy (E, phase 0) and dying (E′, phase 1) egg chambers look normal but later phase egg chambers (E″,E‴, phase 4,5) display defects in engulfment. (F-G‴) Egg chambers expressing bskdsRNA or Mekk1dsRNA in FCs show a similar phenotype. Egg chambers in F-F‴ are phases 0, 1, 3 and 5, and egg chambers in G-G‴ are phases 0, 3, 4 and 5. (H-H‴) Phase 0, 1, 2 and 5 eiger3 egg chambers show little FC enlargement and premature death of the FCs. Scale bar: 50 μm. (I,J) Quantification of the unengulfed area. Data are mean±s.e.m. *P<0.05, ***P<0.001.
Fig. 5.
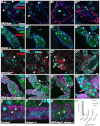
Draper and JNK regulate each other during engulfment. (A-A‴) Egg chambers overexpressing drpr in follicle cells (FCs) show activation of _puc-lacZ (red, arrowheads; flies not starved). Egg chambers stained with DAPI (cyan) and α-DCAD2 to label FC membranes (blue). (B-B‴) drpr_Δ5 egg chambers from starved flies do not express puc-lacZ (red, arrowhead) until late phases of death. Egg chambers are stained as in A and express G71 GFP (green). (C-C‴) FCs that overexpress hepCA (tubulin-GAL80ts/+; UAS-hepCA/GR1 G89) induce Drpr expression (red) in the absence of starvation. Arrowheads indicate FCs and arrows indicate dying nurse cells (NCs). (C‴) Lower magnification image shows widespread induction of Drpr and few late stage egg chambers. (D-D‴) Overexpression of hepCA in drpr_Δ5 background (tubulin-GAL80ts/+; UAS-hepCA/G71; drpr_Δ5 _GR1-GAL4/drpr_Δ5 flies incubated at 29°C, starved) suppresses the drpr phenotype. (D) Healthy egg chamber appears normal. (D′,D″). FCs enlarge (arrowheads) and engulf germline GFP. (D‴) Some egg chambers show a hyper-engulfment phenotype, where FCs (arrowhead) engulf intact NCs (arrow). Scale bar: 50 μm. (E-G) Enlargements of FCs from Fig. 2D′ (E), Fig. 2E' (F) and Fig. 5D′ (G) show that control and _UAS-hepCA; drpr_Δ5 FCs engulf GFP (arrowheads) whereas _drpr_Δ5 FCs do not (F, arrow). Scale bars: 50 μm. (H) Quantification of engulfment as in Fig. 2J for _drpr_Δ5 (_n_=128) and _UAS-hepCA; drpr_Δ5 (_n_=745). Egg chambers from flies incubated at 29°C.
Fig. 6.
Caspase activity is required for proper engulfment but is not required for upregulation of Drpr or JNK activity. (A-F) Caspase mutant egg chambers stained with DAPI (cyan), α-Drpr (yellow) and α-Dlg (magenta). (A′-F′) Drpr staining only (white). (A-C′) Egg chambers from three homozygous alleles of dcp-1 (dcp-1prev1, dcp-12 and dcp-13) show Drpr upregulation in the follicle cells (FCs) when starved. However, most FCs fail to enlarge and display thinning out of their membranes (arrowheads). (D,D′) Egg chambers overexpressing diap1 in the germline (NGT/UASp-diap1; nanos-GAL4/+; starved flies) show Drpr upregulation. FCs fail to enlarge and display thinning out of their membranes (arrowhead). (E-F′) Egg chambers from unstarved flies overexpressing full-length dcp-1 [_nanos-Gal4-tubulin (NGT)/UASp-fl-dcp-1; nanos-GAL4/+_] in the germline show germline death (arrow) but delays in engulfment and Drpr induction (compare E′,F′ with Fig. 2B,C). (G-I′) Caspase mutant egg chambers stained with α-β-gal to detect puc-lacZ (red) and DAPI (cyan). (G,G′) Egg chambers from starved flies overexpressing diap1 in the germline (NGT/UASp-diap1; nanos-GAL4/puc-lacZ) show an induction in puc-lacZ. G′ is probably a later phase egg chamber than G because of the FC loss. (H-I′) Germline overexpression of truncated dcp-1 (nanos-GAL4 UASp-tdcp-1/puc-lacZ) in the absence of starvation leads to death of the germline with delayed puc-lacZ expression. The same egg chamber is shown in H-I' with the red channel only shown in H',I'. I,I' is a later phase egg chamber than H,H' based on nuclear morphology. Arrow in H indicates condensed nurse cell nucleus. Scale bar: 50 μm.
Fig. 7.
Model for Draper-JNK circuit in engulfing follicle cells. Drpr (black rectangles) recognizes an unknown ligand(s) (purple circle) on germline cells, leading to its initial activation in follicle cells (FCs). Drpr becomes phosphorylated by Src kinase and physically interacts with Shark. Shark activates Rac1 and leads to cytoskeletal changes and activation of Basket (JNK). The phosphorylation of Basket leads to the activation of the transcription factor AP-1, which translocates to the nucleus and activates its downstream target, puckered. Additionally, AP-1 is proposed to transcriptionally activate drpr, leading to enrichment of Drpr on the membrane and other engulfment genes. Our data suggest that the caspase Dcp-1 activates an independent pathway that contributes to engulfment.
Similar articles
- Control of non-apoptotic nurse cell death by engulfment genes in Drosophila.
Timmons AK, Mondragon AA, Meehan TL, McCall K. Timmons AK, et al. Fly (Austin). 2017 Apr 3;11(2):104-111. doi: 10.1080/19336934.2016.1238993. Epub 2016 Sep 29. Fly (Austin). 2017. PMID: 27686122 Free PMC article. - Polarization of the epithelial layer and apical localization of integrins are required for engulfment of apoptotic cells in the Drosophila ovary.
Meehan TL, Kleinsorge SE, Timmons AK, Taylor JD, McCall K. Meehan TL, et al. Dis Model Mech. 2015 Dec;8(12):1603-14. doi: 10.1242/dmm.021998. Epub 2015 Sep 22. Dis Model Mech. 2015. PMID: 26398951 Free PMC article. - Scrambled Eggs: Apoptotic Cell Clearance by Non-Professional Phagocytes in the Drosophila Ovary.
Serizier SB, McCall K. Serizier SB, et al. Front Immunol. 2017 Nov 29;8:1642. doi: 10.3389/fimmu.2017.01642. eCollection 2017. Front Immunol. 2017. PMID: 29238344 Free PMC article. Review. - Components of the Engulfment Machinery Have Distinct Roles in Corpse Processing.
Meehan TL, Joudi TF, Timmons AK, Taylor JD, Habib CS, Peterson JS, Emmanuel S, Franc NC, McCall K. Meehan TL, et al. PLoS One. 2016 Jun 27;11(6):e0158217. doi: 10.1371/journal.pone.0158217. eCollection 2016. PLoS One. 2016. PMID: 27347682 Free PMC article. - Clearance of apoptotic corpses.
Fullard JF, Kale A, Baker NE. Fullard JF, et al. Apoptosis. 2009 Aug;14(8):1029-37. doi: 10.1007/s10495-009-0335-9. Apoptosis. 2009. PMID: 19291407 Review.
Cited by
- Understanding the diversity and dynamics of in vivo efferocytosis: Insights from the fly embryo.
Heron R, Amato C, Wood W, Davidson AJ. Heron R, et al. Immunol Rev. 2023 Oct;319(1):27-44. doi: 10.1111/imr.13266. Epub 2023 Aug 17. Immunol Rev. 2023. PMID: 37589239 Free PMC article. Review. - Polar cell fate stimulates Wolbachia intracellular growth.
Kamath AD, Deehan MA, Frydman HM. Kamath AD, et al. Development. 2018 Mar 23;145(6):dev158097. doi: 10.1242/dev.158097. Development. 2018. PMID: 29467241 Free PMC article. - Somatic support cells regulate germ cell survival through the Baz/aPKC/Par6 complex.
Brantley SE, Fuller MT. Brantley SE, et al. Development. 2019 Apr 15;146(8):dev169342. doi: 10.1242/dev.169342. Development. 2019. PMID: 30918053 Free PMC article. - Phagocyte Responses to Cell Death in Flies.
Davidson AJ, Wood W. Davidson AJ, et al. Cold Spring Harb Perspect Biol. 2020 Apr 1;12(4):a036350. doi: 10.1101/cshperspect.a036350. Cold Spring Harb Perspect Biol. 2020. PMID: 31501193 Free PMC article. Review. - Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons.
Tasdemir-Yilmaz OE, Freeman MR. Tasdemir-Yilmaz OE, et al. Genes Dev. 2014 Jan 1;28(1):20-33. doi: 10.1101/gad.229518.113. Epub 2013 Dec 20. Genes Dev. 2014. PMID: 24361692 Free PMC article.
References
- Adachi-Yamada T., Nakamura M., Irie K., Tomoyasu Y., Sano Y., Mori E., Goto S., Ueno N., Nishida Y., Matsumoto K. (1999). p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis. Mol. Cell. Biol. 19, 2322-2329 - PMC - PubMed
- Birge R. B., Ucker D. S. (2008). Innate apoptotic immunity: the calming touch of death. Cell Death Differ. 15, 1096-1102 - PubMed
- Cuttell L., Vaughan A., Silva E., Escaron C. J., Lavine M., Van Goethem E., Eid J. P., Quirin M., Franc N. C. (2008). Undertaker, a Drosophila Junctophilin, links Draper-mediated phagocytosis and calcium homeostasis. Cell 135, 524-534 - PubMed
- Erwig L. P., Henson P. M. (2008). Clearance of apoptotic cells by phagocytes. Cell Death Differ. 15, 243-250 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials