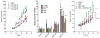Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice - PubMed (original) (raw)
. 2012 Nov 15;72(22):5790-800.
doi: 10.1158/0008-5472.CAN-12-0818. Epub 2012 Sep 19.
Uma T Shankavaram, Mahadev Rao, Nicole Datrice, Scott Atay, Marta Aparicio, Kevin A Camphausen, Pedro M Fernández-Salguero, Han Chang, Pinpin Lin, David S Schrump, Stavros Garantziotis, Frank Cuttitta, Enrique Zudaire
Affiliations
- PMID: 22993405
- PMCID: PMC4340077
- DOI: 10.1158/0008-5472.CAN-12-0818
Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice
Sergio Portal-Nuñez et al. Cancer Res. 2012.
Abstract
Cigarette smoking (CS) is a leading cause of death worldwide. The aryl hydrocarbon receptor (AHR) is partially responsible for tobacco-induced carcinogenesis although the underlying mechanisms involving early effector genes have yet to be determined. Here, we report that adrenomedullin (ADM) significantly contributes to the carcinogenicity of tobacco-activated AHR. CS and AHR activating ligands induced ADM in vitro and in vivo but not in AHR-deficient fibroblasts and mice. Ectopic transfection of AHR rescued ADM expression in AHR(-/-) fibroblasts whereas AHR blockage with siRNA in wild type cells significantly decreased ADM expression. AHR regulates ADM expression through two intronic xenobiotic response elements located close to the start codon in the ADM gene. Using tissue microarrays we showed that ADM and AHR were coupregulated in lung tumor biopsies from smoker patients. Microarray meta-analysis of 304 independent microarray experiments showed that ADM is elevated in smokers and smokers with cancer. In addition, ADM coassociated with a subset of AHR responsive genes and efficiently differentiated patients with lung cancer from nonsmokers. In a novel preclinical model of CS-induced tumor progression, host exposure to CS extracts significantly elevated tumor ADM although systemic treatment with the ADM antagonist NSC16311 efficiently blocked tobacco-induced tumor growth. In conclusion, ADM significantly contributes the carcinogenic effect of AHR and tobacco combustion products. We suggest that therapeutics targeting the AHR/ADM axis may be of clinical relevance in the treatment of tobacco-induced pulmonary malignancies.
©2012 AACR.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
Figure 1
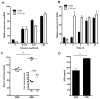
Tobacco smoke induces ADM expression and activates the AHR pathway in lung cancer cells. Exposure of A549 cells to CSE resulted in induction of ADM and CYP1A1 mRNA expression in a dose (A) and time (B) dependent manner. (C) CSE significantly enhanced transactivation of a luciferase reporter under the control of the ADM promoter (pGL3P-AM2575) and a XRE reporter control plasmid (pGudLuc; C insert) in A549 cells. (D) Elevated levels of ADM protein were found in conditioned media of A549 cells exposed to CSE.
Figure 2
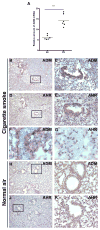
ADM is elevated in lungs of mice exposed to CS. (A) Expression of ADM mRNA is elevated in lungs of mice exposed to CS compared to animals exposed to normal air. Strong ADM (B, C) and AHR (D, E) immunoreactivity colocalized in the epithelium of bronchioles of mice exposed to CS (x100). ADM expression was also elevated in the vascular endothelium of the lung in mice upon exposure to CS (F), although AHR was undetectable in the same vessels (G) (x600). In mice exposed to normal air, ADM immunoreactivity was localized exclusively to some muscle fibers around the bronchioles (H, I) and no AHR staining was detected (J, K) (x100).
Figure 3
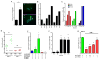
AHR activation results in induction of ADM expression in vitro and in vivo. (A) Increased ADM mRNA and protein was observed in A549 cells after exposure to 3MC for 24h. (B) Exposure of cancer cells from different anatomical origins to TCDD resulted in enhanced ADM mRNA levels, (C) which was found to be time dependent in breast and lung cells lines. (D) AHR null fibroblasts showed no increase in ADM transactivation after exposure to CSE (0.06 puffs/ml for 24h) as opposed to isogenic AHR+/+ cells. A marked increase in basal ADM transactivation was observed between untreated AHR+/+ and AHR−/− cells. (E) Forced co-expression of AHR and ARNT was required in A549 cells to induce upregulation of ADM mRNA. Consistently, transient transfection of two different siRNA for AHR resulted in significant blockage of ADM expression. (F) AHR-ARNT dependent stimulation of ADM expression was shown to be time dependent. (G) Low ADM transactivation driven by the complete ADM promoter (pGL3P-AM2725) in AHR null cells was restored by ectopic expression of AHR in a dose dependent manner.
Figure 4
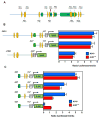
Two intronic XREs are responsible for transactivation of ADM by AHR. (A) 12 putative XREs (orange ovals) were found across the entire genomic sequence of the ADM gene. (B) The two XREs contained in the first intron of the ADM gene are largely responsible for its transactivation in A549 cells and AHR+/+ fibroblasts exposed to CSE (0.06 puffs/ml). (C) Single mutation of the XREs present in this region results in significant diminution of ADM transactivation in both A549 and AHR wild type cells exposed to CSE (0.06 puffs/ml). Mutation of both XREs further impedes ADM transactivation. Exons are indicated green boxes.
Figure 5
ADM drives tobacco-induced tumor progression. (A) Dose dependent tumor growth response to systemic injections of increasing concentrations of CSE. (B) Dose dependent gene expression of ADM and AHR responsive genes in xenograft tumors exposed to increasing concentrations of CSE (0.06–0.18 mg tar). (C) Blockage of ADM with the small molecule antagonist NSC16311 inhibits CSE-induced tumor growth. Treatments with NSC16311 started two weeks after injection of tumor cells (red arrow).
Figure 6
ADM and AHR are co-upregulated in patient lung tumors. A–F, immunodetection of AHR (A,C,E) and ADM (B,D,F) in serial sections of lung cancer biopsies (100x for panoramic images and 600x for inserts).
Figure 7
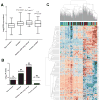
ADM and AHR target genes are coassociated and efficiently differentiate smoking-induced cancer and non-smokers in clinical sample microarrays. (A) Comparison of ADM gene expression in non smokers, and various smoker groups as indicated. The category smoker includes group of smokers who do not have cancer. P value from t-test analysis between non smokers and each of the smoker groups is indicated on top of each bar. (B) Bar graph of –log10 Fisher test P values depicting the enrichment scores of AHR module (479 genes) in each of the groups. The number of genes co-expressed with ADM gene (percentage enrichment in parentheses) is shown on top of each bar (C). Unsupervised clustering of expression of AHR target genes co-associated with ADM in non smoker (red), smoker without cancer (green), smoker with cancer (brown) and former smoker (blue) groups. Rows represent genes and columns represent sample classes.
Similar articles
- AHR activated placental adrenomedullin: A plausible factor in smoke-induced preeclampsia protection.
Marbrey MW, Kistner B, Douglas ES, Caron KM. Marbrey MW, et al. Placenta. 2025 Jun 26;167:175-180. doi: 10.1016/j.placenta.2025.05.013. Epub 2025 May 15. Placenta. 2025. PMID: 40408837 - Aryl hydrocarbon receptor protects lung adenocarcinoma cells against cigarette sidestream smoke particulates-induced oxidative stress.
Cheng YH, Huang SC, Lin CJ, Cheng LC, Li LA. Cheng YH, et al. Toxicol Appl Pharmacol. 2012 Mar 15;259(3):293-301. doi: 10.1016/j.taap.2012.01.005. Epub 2012 Jan 16. Toxicol Appl Pharmacol. 2012. PMID: 22273977 - Aryl Hydrocarbon Receptor: Its Regulation and Roles in Transformation and Tumorigenesis.
Che X, Dai W. Che X, et al. Curr Drug Targets. 2019;20(6):625-634. doi: 10.2174/1389450120666181109092225. Curr Drug Targets. 2019. PMID: 30411679 Review. - New Insight into the Role of AhR in Lung Carcinogenesis.
Akhmetova DA, Kozlov VV, Gulyaeva LF. Akhmetova DA, et al. Biochemistry (Mosc). 2022 Nov;87(11):1219-1225. doi: 10.1134/S0006297922110013. Biochemistry (Mosc). 2022. PMID: 36509717 Review.
Cited by
- AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma.
Zhu Q, Ma Y, Liang J, Wei Z, Li M, Zhang Y, Liu M, He H, Qu C, Cai J, Wang X, Zeng Y, Jiao Y. Zhu Q, et al. Signal Transduct Target Ther. 2021 Aug 9;6(1):299. doi: 10.1038/s41392-021-00713-1. Signal Transduct Target Ther. 2021. PMID: 34373448 Free PMC article. - ASXL3 Is a Novel Pluripotency Factor in Human Respiratory Epithelial Cells and a Potential Therapeutic Target in Small Cell Lung Cancer.
Shukla V, Rao M, Zhang H, Beers J, Wangsa D, Wangsa D, Buishand FO, Wang Y, Yu Z, Stevenson HS, Reardon ES, McLoughlin KC, Kaufman AS, Payabyab EC, Hong JA, Zhang M, Davis S, Edelman D, Chen G, Miettinen MM, Restifo NP, Ried T, Meltzer PA, Schrump DS. Shukla V, et al. Cancer Res. 2017 Nov 15;77(22):6267-6281. doi: 10.1158/0008-5472.CAN-17-0570. Epub 2017 Sep 21. Cancer Res. 2017. PMID: 28935813 Free PMC article. - Impact of AHR Ligand TCDD on Human Embryonic Stem Cells and Early Differentiation.
Teino I, Matvere A, Pook M, Varik I, Pajusaar L, Uudeküll K, Vaher H, Trei A, Kristjuhan A, Org T, Maimets T. Teino I, et al. Int J Mol Sci. 2020 Nov 28;21(23):9052. doi: 10.3390/ijms21239052. Int J Mol Sci. 2020. PMID: 33260776 Free PMC article. - Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target.
Safe S, Lee SO, Jin UH. Safe S, et al. Toxicol Sci. 2013 Sep;135(1):1-16. doi: 10.1093/toxsci/kft128. Epub 2013 Jun 14. Toxicol Sci. 2013. PMID: 23771949 Free PMC article. Review. - Circulating Levels of the Cardiovascular Biomarkers ST2 and Adrenomedullin Predict Outcome within a Randomized Phase III Lung Cancer Trial (RASTEN).
Gezelius E, Bendahl PO, Gallo W, de Oliveira KG, Ek L, Bergman B, Sundberg J, Melander O, Belting M. Gezelius E, et al. Cancers (Basel). 2022 Mar 3;14(5):1307. doi: 10.3390/cancers14051307. Cancers (Basel). 2022. PMID: 35267617 Free PMC article.
References
- Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–9. - PubMed
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
- Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, et al. Tobacco and cancer: recent epidemiological evidence. Journal of the National Cancer Institute. 2004;96:99–106. - PubMed
- Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol. 2005;23:3243–56. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases