The DEAD-box helicase eIF4A: paradigm or the odd one out? - PubMed (original) (raw)
Review
The DEAD-box helicase eIF4A: paradigm or the odd one out?
Alexandra Z Andreou et al. RNA Biol. 2013 Jan.
Abstract
DEAD-box helicases catalyze the ATP-dependent unwinding of RNA duplexes. They share a helicase core formed by two RecA-like domains that carries a set of conserved motifs contributing to ATP binding and hydrolysis, RNA binding and duplex unwinding. The translation initiation factor eIF4A is the founding member of the DEAD-box protein family, and one of the few examples of DEAD-box proteins that consist of a helicase core only. It is an RNA-stimulated ATPase and a non-processive helicase that unwinds short RNA duplexes. In the catalytic cycle, a series of conformational changes couples the nucleotide cycle to RNA unwinding. eIF4A has been considered a paradigm for DEAD-box proteins, and studies of its function have revealed the governing principles underlying the DEAD-box helicase mechanism. However, as an isolated helicase core, eIF4A is rather the exception, not the rule. Most helicase modules in other DEAD-box proteins are modified, some by insertions into the RecA-like domains, and the majority by N- and C-terminal appendages. While the basic catalytic function resides within the helicase core, its modulation by insertions, additional domains or a network of interaction partners generates the diversity of DEAD-box protein functions in the cell. This review summarizes the current knowledge on eIF4A and its regulation, and discusses to what extent eIF4A serves as a model DEAD-box protein.
Keywords: ATP-driven conformational changes; DEAD-box helicase; RNA unwinding; translation initiation.
Figures
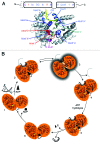
Figure 1. RNA unwinding by DEAD-box proteins. (A) Schematic depiction of DEAD-box protein architecture (top) and structure of eIF4A-III (PDB-ID 2j0s) in complex with single-stranded RNA (yellow) and ADPNP (green). The helicase core is formed by two RecA-like domains that carry the conserved helicase signature motifs. Motifs involved in ATP binding and hydrolysis are depicted in red hues, motifs involved in RNA binding in blue hues, and motifs involved in coupling of ATP hydrolysis to duplex separation in purple. In many DEAD-box proteins, the helicase core is flanked by N- and C-terminal extensions. (B) Conformational changes in the catalytic cycle of DEAD-box proteins. In the absence of RNA or ATP, the helicase core of DEAD-box proteins exists in an open conformation, in which the cleft between the two RecA-like domains is open, and there are no inter-domain contacts formed (1). Cooperative binding of ATP and RNA stabilizes a closed conformation of the helicase core (2), with a network of interactions between the conserved helicase motifs, and with the bound ATP and RNA. A rearrangement of hitherto unidentified nature leads to the formation of an activated complex (3). The RNA duplex is locally destabilized by deformation, and the first strand can dissociate (4). The core retains the closed conformation upon ATP hydrolysis (5) and returns to the open conformation upon phosphate release (6). Opening disrupts the bipartite RNA binding site, and hence leads to release of the second RNA strand (6). After ADP dissociation, the helicase core can undergo further catalytic cycles. Reprinted from Methods in Enzymology 511, Alexandra Z. Andreou and Dagmar Klostermeier, Conformational Changes of DEAD-Box Helicases Monitored by Single Molecule Fluorescence Resonance Energy Transfer, pages 75–110, Copyright 2012, with permission from Elsevier.
Figure 2. Domain organization of the mammalian translation initiation factor eIF4A-I and its interaction factors eIF4B, eI4H, eIF4E and eIF4G. For eIF4A-I, the N-terminal RecA-like domain is depicted in red, and the C-terminal in yellow. For eIF4B, the RNA recognition motif (RRM) is shown in cyan, the DRYG repeat domain in black, and the basic domain (BD) in yellow. For eIF4H, the RRM is also depicted in cyan. The eIF4E-like domain of eIF4E is depicted in violet. For eIF4G, the binding domain for the poly-A binding protein (PABP) is indicated in turquoise, for eIF4E in green, for eIF3 in blue, for Mnk1 in purple and the two binding sites for eIF4A-I, i.e. eIF4G-M and eIF4G-C, in orange. Structurally characterized domains are depicted in cartoon representation using the same color code. Upper left: eIF4A-I in the ligand-free, open conformation (PDB-ID 1fuu), upper right: eIF4B RRM (PDB-ID: 2j76), bottom left: eIF4E in complex with eIF4G (residues 393–490; PDB-ID: 1rf8), bottom right: eIF4A-I in complex with eIF4G-M (PDB-ID: 2vso).
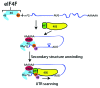
Figure 3. Simplified model of eukaryotic translation initiation. The heterotrimeric protein complex eIF4F, which consists of the DEAD-box RNA helicase eIF4A-I, the scaffolding protein eIF4G and the cap binding protein eIF4E, binds to the m7G cap at the 5′-end of the mRNA. The interaction between eIF4G and the poly-A binding protein (PABP) promotes mRNA circularization. eIF4A-I unwinding activity is enhanced by the interaction with eIF4B (or eIF4H). Interaction between eIF4G and ribosome bound eIF3 may facilitate 43S ribosome complex recruitment to the mRNA. eIF4A-I, in complex with its binding partners, unwinds secondary structures in the 5′-untranslated region (UTR) of the mRNA, and allows for scanning of the 43S complex toward the AUG initiation codon.
Figure 4. Domain organization of eIF4A-III and its interaction partners MLN51, Magoh, and Y14 in the exon junction complex. Domain organization of the human exon junction complex components eIF4A-III, Y14, Magoh and MLN51. The N-terminal RecA-domain of eIF4A-III is depicted in teal, the C-terminal domain in brown, the RRM of Y14 in pink, Magoh in cyan and the Btz domain of MLN51 in yellow. Bottom: Structure of the exon junction complex in cartoon representation using the same color code (PDB ID: 2j0s). Bound RNA is depicted in red.
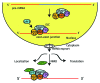
Figure 5. eIF4A-III, the exon junction complex and its link to pre-mRNA splicing and mRNA fate. eIF4A-III is a core component of the exon junction complex (EJC) together with the Y14-Magoh heterodimer and MLN51. The complex is deposited ~25 nucleotides upstream of the exon-exon junction during splicing and remains bound during nuclear export of the RNA into the cytoplasm. The EJC plays a role in mRNA localization, translation and nonsense mediated decay (NMD). It interacts with one of the NMD effectors, Up-frameshift protein 3 (Upf3), in the nucleus. Upf3 then anchors Upf2 in the cytoplasm. The triggering step of NMD upon Upf1 binding to the EJC-Upf3-Upf2 complex is not shown. A prerequisite for activation of the NMD pathway is the translation termination at a stop codon that is 30 nucleotides upstream of an EJC.
Similar articles
- Duplex unwinding and ATPase activities of the DEAD-box helicase eIF4A are coupled by eIF4G and eIF4B.
Özeş AR, Feoktistova K, Avanzino BC, Fraser CS. Özeş AR, et al. J Mol Biol. 2011 Sep 30;412(4):674-87. doi: 10.1016/j.jmb.2011.08.004. Epub 2011 Aug 5. J Mol Biol. 2011. PMID: 21840318 Free PMC article. - Structural basis for RNA-duplex recognition and unwinding by the DEAD-box helicase Mss116p.
Mallam AL, Del Campo M, Gilman B, Sidote DJ, Lambowitz AM. Mallam AL, et al. Nature. 2012 Oct 4;490(7418):121-5. doi: 10.1038/nature11402. Epub 2012 Sep 2. Nature. 2012. PMID: 22940866 Free PMC article. - The mechanism of ATP-dependent RNA unwinding by DEAD box proteins.
Hilbert M, Karow AR, Klostermeier D. Hilbert M, et al. Biol Chem. 2009 Dec;390(12):1237-50. doi: 10.1515/BC.2009.135. Biol Chem. 2009. PMID: 19747077 Review. - Fluorescence methods in the investigation of the DEAD-box helicase mechanism.
Andreou AZ, Klostermeier D. Andreou AZ, et al. Exp Suppl. 2014;105:161-92. doi: 10.1007/978-3-0348-0856-9_8. Exp Suppl. 2014. PMID: 25095995 Review.
Cited by
- Novel eIF4A1 inhibitors with anti-tumor activity in lymphoma.
Kayastha F, Herrington NB, Kapadia B, Roychowdhury A, Nanaji N, Kellogg GE, Gartenhaus RB. Kayastha F, et al. Mol Med. 2022 Sep 4;28(1):101. doi: 10.1186/s10020-022-00534-0. Mol Med. 2022. PMID: 36058921 Free PMC article. - Upstream Open Reading Frames Located in the Leader of Protein Kinase Mζ mRNA Regulate Its Translation.
Bal NV, Susorov D, Chesnokova E, Kasianov A, Mikhailova T, Alkalaeva E, Balaban PM, Kolosov P. Bal NV, et al. Front Mol Neurosci. 2016 Oct 13;9:103. doi: 10.3389/fnmol.2016.00103. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27790092 Free PMC article. - Targeting translation initiation by synthetic rocaglates for treating MYC-driven lymphomas.
Zhang X, Bi C, Lu T, Zhang W, Yue T, Wang C, Tian T, Zhang X, Huang Y, Lunning M, Hao X, Brown LE, Devine WG, Vose J, Porco JA Jr, Fu K. Zhang X, et al. Leukemia. 2020 Jan;34(1):138-150. doi: 10.1038/s41375-019-0503-z. Epub 2019 Jun 6. Leukemia. 2020. PMID: 31171817 Free PMC article. - Bi-directional ribosome scanning controls the stringency of start codon selection.
Gu Y, Mao Y, Jia L, Dong L, Qian SB. Gu Y, et al. Nat Commun. 2021 Nov 15;12(1):6604. doi: 10.1038/s41467-021-26923-3. Nat Commun. 2021. PMID: 34782646 Free PMC article. - Lifelong companions: RNA helicases and their roles in RNA metabolism.
Klostermeier D. Klostermeier D. RNA Biol. 2013 Jan;10(1):2-3. doi: 10.4161/rna.23500. Epub 2013 Jan 1. RNA Biol. 2013. PMID: 23353572 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous