Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes - PubMed (original) (raw)
Review
Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes
John R Giudicessi et al. Transl Res. 2013 Jan.
Abstract
Mutations in genes encoding ion channel pore-forming α-subunits and accessory β-subunits as well as intracellular calcium-handling proteins that collectively maintain the electromechanical function of the human heart serve as the underlying pathogenic substrate for a spectrum of sudden cardiac death (SCD)-predisposing heritable cardiac arrhythmia syndromes, including long QT syndrome (LQTS), short QT syndrome (SQTS), Brugada syndrome (BrS), and catecholaminergic polymorphic ventricular tachycardia (CPVT). Similar to many Mendelian disorders, the cardiac "channelopathies" exhibit incomplete penetrance, variable expressivity, and phenotypic overlap, whereby genotype-positive individuals within the same genetic lineage assume vastly different clinical courses as objectively assessed by phenotypic features such electrocardiographic abnormalities and number/type of cardiac events. In this Review, we summarize the current understanding of the global architecture of complex electrocardiographic traits such as the QT interval, focusing on the role of common genetic variants in the modulation of ECG parameters in health and the environmental and genetic determinants of incomplete penetrance and variable expressivity in the heritable cardiac arrhythmia syndromes most likely to be encountered in clinical practice.
Copyright © 2013 Mosby, Inc. All rights reserved.
Figures
Figure 1
Penetrance and expressivity in a generic heritable cardiac arrhythmia syndrome. a | Representative multigenerational pedigree displaying complete penetrance (100%) for the electrocardiographic and arrhythmic hallmarks of the disease. b | Representative multigenerational pedigree displaying incomplete penetrance (33%) for the electrocardiographic and arrhythmic hallmarks of the disease. c | Representative multigenerational pedigree displaying incomplete penetrance (66%) and variable expressivity as some individuals display the electrocardiographic hallmarks of the disease without symptomatology.
Figure 2
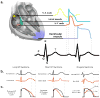
Electrical activity of the heart in health and disease. a | Schematic representation of the normal cardiac conduction system and the correlation between the action potentials of cardiomyocytes in distinct areas of the heart and the surface electrocardiogram. b | Schematic representation of a normal ECG (black) and typical ECGs for patients with LQTS (left, red), SQTS (middle, red), and BrS (right, red). c | Tracings of normal ventricular action potentials (black) and tracings that display epicardial action-potential prolongation in LQTS (left, red), action-potential abbreviation in SQTS (middle, red), and the transmural gradient between the epicardial action potential (right, solid red line) and endocardial action-potential (right, dotted red line) that results in the inscription of the J-wave in BrS. BrS, Brugada syndrome; ECG, electrocardiogram; LQT, long QT syndrome; SQT, short-QT syndrome. Reproduced in part from Wilde, A. A. & Bezzina, C. R. Genetics of cardiac arrhythmias. Heart 91(10), 1352–1358 © 2005, with permission from BMJ Publishing Group Ltd.
Figure 3
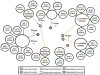
Current-centric classification of heritable cardiac arrhythmia syndromes. The clinical phenotypes resulting from the abnormal ventricular cardiac action potential depolarization (orange) or repolarization (purple) are grouped according to the specific current perturbed by an underlying genetic defect. Blue circles represent loss-of-function mutations to the specified current, whereas green circles represent a gain-of-function. Solid lines indicate those disorders that are autosomal dominant, whereas dashed lines indicate those disorders that are autosomal recessive. Abbreviations: BrS, Brugada syndrome; LQT, long QT syndrome; ICa,L, L-type calcium current; IK1, inwardly rectifying current; IKATP, ATP-sensitive potassium current; IKr, rapid component of the delayed rectifier potassium current; IKs, slow-component of the delayed rectifier potassium current; Ito, transient outward potassium current; INa, cardiac sodium current; SQT, short-QT syndrome.
Figure 4
QTc distribution in health and disease. The distribution of QTc values in health was derived from nearly 80,000 healthly adult males and females.[81] The distribution of QTc values in LQTS were derived from all patients with genetically proven LQTS evaluated in the Mayo Clinic Long QT Syndrome Clinic and the distribution of QTc values in SQTS from cases identified in a recent meta-analysis[82]. Permission obtained from Wolters Kluwer Health © Taggart, N.W. et al. Diagnostic miscues in congenital long QT syndrome. Circulation 115(20), 2613–2620 (2007).
Figure 5
Genetic architecture underlying the complex phenotyptes associated with perturbed myocardial repolarization/depolarization. A spectrum of genetic variation underlies the genetic architecture of myocardial repolarization/depolarization abnormalities. At the severe (red) end of the spectrum are extremely rare (<1% minor allele frequency) mutations that strongly perturb the cardiac action potential and typically result in monogenic disorders such as LQTS and BrS. In the middle of the spectrum (yellow) are rare variants that moderately perturb the cardiac action potential, but often require a second hit (e.g. genetic modifier, QT-prolonging drug, etc.) to myocardial repolarization/depolarization to produce the electrocardiographic or arrhythmic manifestations associated with monogenic disease. Lastly, at the benign (green) end of the spectrum are common (>5% minor allele frequency) variants that weakly perturb the cardiac action potential and only contribute to the electrocardiographic and arrhythmic manifestations of disease in rare circumstances when multiple hits (genetic and environmental) to myocardial repolarization/depolarization are present.
Figure 6
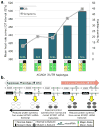
The allele-specific effects and hypothesized mechanism of LQT1 disease-modifying SNPs in the 3′UTR of KCNQ1. a | Summarized allele-specific effects of SNPs rs2519184, rs8234, and rs10798 on the heart-rate corrected QT interval (dark gray bars) and occurrence of symptoms (light gray line) in the combined Mayo Clinic and Academic Medical Center LQT1 cohort. b | Hypothesized microRNA-mediated mechanism whereby the minor alleles (dark grey boxes) of SNPs in the 3′UTR of KCNQ1 alter the stoichiometric assembly of wild-type (white circles) and mutant (dark circles) Kv7.1 α-subunits derived from mutant (black) and normal (white) KCNQ1 alleles, respectively. Numbers above genotype denote group sizes. ** p ≤ 0.05 for average QTc and % cardiac events in comparison to the wild-type 3′UTR haplotype (NGAA/MGAA), * p ≤ 0.05 for average QTc compared to the wild-type 3′UTR haplotype (NGAA/MGAA), N, normal KCNQ1 allele, M, mutant KCNQ1 allele. Reproduced from Amin, A.S., Giudicessi, J.R., and Tijsen, A.J., et al. Variants in the 3′ untranslated region of the _KCNQ1_-encoded Kv7.1 potassium channel modify disease severity in patients with type 1 long QT syndrome in an allele-specific manner. Eur Heart J 33(6), 714–723 © 2012 with permission from Oxford University Press.
Similar articles
- Role of pharmacotherapy in cardiac ion channelopathies.
El-Sherif N, Boutjdir M. El-Sherif N, et al. Pharmacol Ther. 2015 Nov;155:132-42. doi: 10.1016/j.pharmthera.2015.09.002. Epub 2015 Sep 12. Pharmacol Ther. 2015. PMID: 26376080 Review. - Role of pharmacotherapy in cardiac ion channelopathies.
El-Sherif N, Pedalino R, Himel H 4th. El-Sherif N, et al. Curr Vasc Pharmacol. 2009 Jul;7(3):358-66. doi: 10.2174/157016109788340794. Curr Vasc Pharmacol. 2009. PMID: 19601860 Review. - Arrhythmogenic hereditary syndromes: Brugada Syndrome, long QT syndrome, short QT syndrome and CPVT.
Schimpf R, Veltmann C, Wolpert C, Borggrefe M. Schimpf R, et al. Minerva Cardioangiol. 2010 Dec;58(6):623-36. Minerva Cardioangiol. 2010. PMID: 21135804 Review. - Channelopathies, genetic testing and risk stratification.
Wilde AAM, Amin A. Wilde AAM, et al. Int J Cardiol. 2017 Jun 15;237:53-55. doi: 10.1016/j.ijcard.2017.03.063. Epub 2017 Mar 18. Int J Cardiol. 2017. PMID: 28343764 Review.
Cited by
- Calcium Revisited: New Insights Into the Molecular Basis of Long-QT Syndrome.
Giudicessi JR, Ackerman MJ. Giudicessi JR, et al. Circ Arrhythm Electrophysiol. 2016 Jul;9(7):e002480. doi: 10.1161/CIRCEP.116.002480. Circ Arrhythm Electrophysiol. 2016. PMID: 27390209 Free PMC article. Review. No abstract available. - Mental Resilience, Mood, and Quality of Life in Young Adults with Self-Reported Impaired Wound Healing.
Balikji J, Hoogbergen MM, Garssen J, Verster JC. Balikji J, et al. Int J Environ Res Public Health. 2022 Feb 22;19(5):2542. doi: 10.3390/ijerph19052542. Int J Environ Res Public Health. 2022. PMID: 35270235 Free PMC article. - Study of the Effects of Cordia myxa Fruit Extract on Induced Animal Model of Depression in Male Rats.
Jasib Habeeb Y, Mohammed Selman S, Jeafer Mehrath A. Jasib Habeeb Y, et al. Arch Razi Inst. 2022 Aug 31;77(4):1503-1511. doi: 10.22092/ARI.2022.357642.2082. eCollection 2022 Aug. Arch Razi Inst. 2022. PMID: 36883145 Free PMC article. - Apathy-Related Symptoms Appear Early in Parkinson's Disease.
Cohen E, Bay AA, Ni L, Hackney ME. Cohen E, et al. Healthcare (Basel). 2022 Jan 4;10(1):91. doi: 10.3390/healthcare10010091. Healthcare (Basel). 2022. PMID: 35052255 Free PMC article. - Personalized Interpretation and Clinical Translation of Genetic Variants Associated With Cardiomyopathies.
Campuzano O, Fernandez-Falgueras A, Sarquella-Brugada G, Cesar S, Arbelo E, García-Álvarez A, Jordà P, Coll M, Fiol V, Iglesias A, Perez-Serra A, Mates J, Del Olmo B, Ferrer C, Alcalde M, Puigmulé M, Mademont-Soler I, Pico F, Lopez L, Tiron C, Brugada J, Brugada R. Campuzano O, et al. Front Genet. 2019 May 15;10:450. doi: 10.3389/fgene.2019.00450. eCollection 2019. Front Genet. 2019. PMID: 31156706 Free PMC article.
References
- Cerrone M, Priori SG. Genetics of sudden death: focus on inherited channelopathies. Eur Heart J. 2011;32:2109–18. - PubMed
- Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long-QT syndrome: clinical impact. Circulation. 1999;99:529–33. - PubMed
- Scicluna BP, Wilde AA, Bezzina CR. The primary arrhythmia syndromes: same mutation, different manifestations. Are we starting to understand why? J Cardiovasc Electrophysiol. 2008;19:445–52. - PubMed
- Morita H, Wu J, Zipes DP. The QT syndromes: long and short. Lancet. 2008;372:750–63. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical