TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma - PubMed (original) (raw)
TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma
Jieqiong Liu et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Although the role of TGF-β in tumor progression has been studied extensively, its impact on drug delivery in tumors remains far from understood. In this study, we examined the effect of TGF-β blockade on the delivery and efficacy of conventional therapeutics and nanotherapeutics in orthotopic mammary carcinoma mouse models. We used both genetic (overexpression of sTβRII, a soluble TGF-β type II receptor) and pharmacologic (1D11, a TGF-β neutralizing antibody) approaches to block TGF-β signaling. In two orthotopic mammary carcinoma models (human MDA-MB-231 and murine 4T1 cell lines), TGF-β blockade significantly decreased tumor growth and metastasis. TGF-β blockade also increased the recruitment and incorporation of perivascular cells into tumor blood vessels and increased the fraction of perfused vessels. Moreover, TGF-β blockade normalized the tumor interstitial matrix by decreasing collagen I content. As a result of this vessel and interstitial matrix normalization, TGF-β blockade improved the intratumoral penetration of both a low-molecular-weight conventional chemotherapeutic drug and a nanotherapeutic agent, leading to better control of tumor growth.
Conflict of interest statement
Conflict of interest statement: R.K.J. received research grants from Dyax, MedImmune, and Roche; received consultant fees from Dyax, Enlight, Noxxon, and SynDevRx; owns equity in Enlight, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and Board of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors. Y.B. received consultant fees from XTuit. No reagents or funding from these companies were used in these studies.
Figures
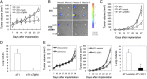
Fig. 1.
Blocking TGF-β signaling inhibits the growth and metastasis of orthotopic mammary carcinoma. (A) Primary tumor growth curves in mice bearing parental (n = 9), mock (n = 8), and sTβRII-transfected (n = 6) MDA-MB-231 cells. *P < 0.0001. (B) Representative BLI of inguinal (In-LN) and axillary (A-LN) lymph nodes (n = 4). Color scale: min, 27; max, 30308. (C and D) Primary tumor growth curves (C; n = 8) and lung metastasis quantification (D; n = 12) in mice bearing parental, mock, and sTβRII-transfected 4T1 cells. (C) *P < 0.0001. (D) *P < 0.005. (E Left and Center) Primary tumor growth curves of MDA-MB-231 (Left) and 4T1 (Center) tumors treated with control IgG (13C4) or anti-TGF-β antibody, 1D11 (n = 8). *P < 0.0001. (Right) Quantification of 4T1 tumor lung metastasis (n = 8). *P < 0.005.
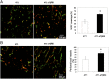
Fig. 2.
Blocking TGF-β signaling increases tumor blood vessel pericyte coverage and perfusion. (A) Representative images and quantification of immunofluorescence double staining for endothelial cells (CD31) and pericytes (NG2) in frozen sections of 4T1 and 4T1–sTβRII tumors. Green, CD31+ staining; red, NG2+ staining; yellow, colocalization of red and green. (B) Representative images and quantification of perfused blood vessels (FITC–lectin) and immunofluorescence staining for endothelial cells (CD31) in frozen sections of 4T1 and 4T1–sTβRII tumors. Green, FITC–lectin-labeled perfused vessels; red, CD31+ staining in nonperfused vessels; yellow, CD31+ staining of perfused vessels. P < 0.001.
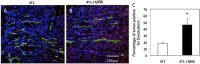
Fig. 3.
Blocking TGF-β signaling improves intratumoral distribution of doxorubicin in orthotopic mammary carcinoma models. (A and B) Representative images of doxorubicin intratumoral distribution in 4T1 (A) and 4T1–sTβRII (B) tumors. Green, FITC–lectin-labeled perfused vessels; red, fluorescent doxorubicin; blue, DAPI. (C) Quantification of the fraction of tumor area positive for doxorubicin (n = 12 sections, with 3 sections per tumor). *P < 0.001.
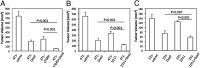
Fig. 4.
Blocking TGF-β signaling decreases collagen I content and improves Doxil tissue penetration. (A) Representative images and quantification of collagen I immunofluorescent staining in 4T1 and 4T1–sTβRII tumors. Red, collagen I staining; blue, DAPI (×20). (B) Representative images and quantification of Doxil intratumoral distribution in 4T1 and 4T1–sTβRII tumors. Green, FITC-lectin labeled perfused vessels; red, fluorescent doxorubicin; blue, DAPI (n = 12 sections, with 3 sections per tumor). *P < 0.001.
Fig. 5.
Blocking TGF-β signaling enhances Doxil efficacy in orthotopic mammary carcinoma models. (A and B) Primary tumor growth of 4T1 and 4T1–sTβRII tumors, with or without Doxil treatment (A) and 4T1 (B) and MDA-MB-231 (C) tumors treated with saline (control), Doxil alone (9 mg/kg, weekly), 1D11 alone (5 mg/kg, three times a week), or combined Doxil and 1D11 (n = 8 in all groups).
Similar articles
- Targeting the Transforming Growth Factor-beta pathway inhibits human basal-like breast cancer metastasis.
Ganapathy V, Ge R, Grazioli A, Xie W, Banach-Petrosky W, Kang Y, Lonning S, McPherson J, Yingling JM, Biswas S, Mundy GR, Reiss M. Ganapathy V, et al. Mol Cancer. 2010 May 26;9:122. doi: 10.1186/1476-4598-9-122. Mol Cancer. 2010. PMID: 20504320 Free PMC article. - An orally active small molecule TGF-beta receptor I antagonist inhibits the growth of metastatic murine breast cancer.
Rausch MP, Hahn T, Ramanathapuram L, Bradley-Dunlop D, Mahadevan D, Mercado-Pimentel ME, Runyan RB, Besselsen DG, Zhang X, Cheung HK, Lee WC, Ling LE, Akporiaye ET. Rausch MP, et al. Anticancer Res. 2009 Jun;29(6):2099-109. Anticancer Res. 2009. PMID: 19528470 Free PMC article. - Effects of TGF-β signaling blockade on human A549 lung adenocarcinoma cell lines.
Xu CC, Wu LM, Sun W, Zhang N, Chen WS, Fu XN. Xu CC, et al. Mol Med Rep. 2011 Sep-Oct;4(5):1007-15. doi: 10.3892/mmr.2011.530. Epub 2011 Jul 1. Mol Med Rep. 2011. PMID: 21725601 - Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis.
Wendt MK, Schiemann WP. Wendt MK, et al. Breast Cancer Res. 2009;11(5):R68. doi: 10.1186/bcr2360. Breast Cancer Res. 2009. PMID: 19740433 Free PMC article. - Parathyroid hormone-related protein and bone metastases.
Guise TA. Guise TA. Cancer. 1997 Oct 15;80(8 Suppl):1572-80. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1572::aid-cncr7>3.3.co;2-d. Cancer. 1997. PMID: 9362424 Review.
Cited by
- Strategies for improving drug delivery: nanocarriers and microenvironmental priming.
Khalid A, Persano S, Shen H, Zhao Y, Blanco E, Ferrari M, Wolfram J. Khalid A, et al. Expert Opin Drug Deliv. 2017 Jul;14(7):865-877. doi: 10.1080/17425247.2017.1243527. Epub 2016 Oct 11. Expert Opin Drug Deliv. 2017. PMID: 27690153 Free PMC article. Review. - Biophysical and epigenetic regulation of cancer stemness, invasiveness and immune action.
Veerasubramanian PK, Trinh A, Akhtar N, Liu WF, Downing TL. Veerasubramanian PK, et al. Curr Tissue Microenviron Rep. 2020 Dec;1(4):277-300. doi: 10.1007/s43152-020-00021-w. Epub 2020 Nov 2. Curr Tissue Microenviron Rep. 2020. PMID: 33817661 Free PMC article. - Exploring the tumor microenvironment with nanoparticles.
Miao L, Huang L. Miao L, et al. Cancer Treat Res. 2015;166:193-226. doi: 10.1007/978-3-319-16555-4_9. Cancer Treat Res. 2015. PMID: 25895870 Free PMC article. Review. - Therapeutic Strategies to Overcome Fibrotic Barriers to Nanomedicine in the Pancreatic Tumor Microenvironment.
Tanaka HY, Nakazawa T, Enomoto A, Masamune A, Kano MR. Tanaka HY, et al. Cancers (Basel). 2023 Jan 24;15(3):724. doi: 10.3390/cancers15030724. Cancers (Basel). 2023. PMID: 36765684 Free PMC article. Review. - Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model.
Zhang J, Miao L, Guo S, Zhang Y, Zhang L, Satterlee A, Kim WY, Huang L. Zhang J, et al. J Control Release. 2014 May 28;182:90-6. doi: 10.1016/j.jconrel.2014.03.016. Epub 2014 Mar 15. J Control Release. 2014. PMID: 24637468 Free PMC article.
References
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46:149–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA126642/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- K99 HL111343/HL/NHLBI NIH HHS/United States
- R01-CA85140/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous