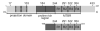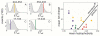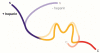Identification of an aggregation-prone structure of tau - PubMed (original) (raw)
. 2012 Oct 10;134(40):16607-13.
doi: 10.1021/ja305206m. Epub 2012 Oct 1.
Affiliations
- PMID: 22998648
- PMCID: PMC3477793
- DOI: 10.1021/ja305206m
Identification of an aggregation-prone structure of tau
Shana Elbaum-Garfinkle et al. J Am Chem Soc. 2012.
Abstract
The aggregation and deposition of normally soluble proteins is the hallmark of several devastating neurodegenerative disorders. For proteins such as tau in Alzheimer's disease and α-synuclein in Parkinson's disease, aggregation involves a transition from an intrinsically disordered monomer to a highly structured fiber. While understanding the role of these proteins in neurodegeneration requires elucidation of the structural basis of self-association, the conformational heterogeneity of disordered proteins makes their structural characterization inherently challenging. Here we use single molecule Förster resonance energy transfer to measure the conformational ensemble of tau in the absence and presence of heparin to identify critical conformational changes relevant to the initiation of aggregation. We find that different domains of tau display distinct conformational properties that are strongly correlated with their degree of disorder and that may relate to their roles in aggregation. Moreover, we observe that heparin binding induces a distinct two-state structural transition in tau characterized by a loss of long-range contacts and a concomitant compaction of the microtubule binding domain. Our results describe a conformational intermediate of tau that precedes the formation of aggregates and could serve as a target for tau-focused therapeutics.
Figures
Figure 1
Tau schematic. The longest full-length tau isoform (top) and the K16 fragment (bottom) are pictured. Regions of interest indicated are: the projection domain, the proline-rich region, and the MTBR (including the four repeats R1-R4). The residues mutated for labeling with fluorescent probes are shown above the schematic. Alternative splicing of two N-terminal exons and one exon in the MTBR give rise to six different tau isoforms (exon boundaries denoted with dashed lines).
Figure 2
Single molecule FRET histograms of eight full-length tau constructs. Representative ETeff histograms are shown for constructs 17-433 (A), 17-291 (B), 17-103 (C), 103-184 (D), 291-433 (E), 322-433 (F), 354-433 (G) and 184-291 (H) in the absence (top panels) and in the presence of 10 μM heparin (bottom panels). The mean measured ETeff (solid line) and the theoretical ETeff, ⟨E⟩, calculated for a model RC (dashed line) of each construct are indicated on the plots (see the manuscript text, SI, and Table 1). In the bottom panels of (A) and (B) the dotted gray line denotes the fit to a standard “zero-peak” to illustrate that the additional width in these distributions are due to a very low ETeff signal.
Figure 3
Charge screening by NaCl. ETeff histograms in 500mM NaCl are pictured for full-length constructs 244-354 (A), 354-433 (B), 17-103 (C) and 17-433 (D) with colored vertical lines indicating the peak position for each construct at 50mM NaCl. (E) Mean net charge as a function of mean hydrophobicity for all constructs; the MTBR and pro line-rich region constructs are in yellow; the C-terminal constructs are in red; the N-terminal constructs are in blue; the end-to-end construct 17-433 is in green, and the full-length protein is in grey.
Figure 4
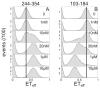
Heparin binding to tau. Titration of heparin into tau results in the appearance of a distinct peak upon binding of heparin. Notably, heparin binding causes compaction in one construct (A: 244-354) and expansion of the other (B: 103-184). The concentration of heparin is noted on each panel. For details on extracted binding curves see SI text and Figure S2.
Figure 5
Model of conformational changes associated with population of aggregation-prone conformational ensemble. Color coding of the regions corresponds to Figure 3: projection domain (blue), MTBR (yellow), C-terminus (red) for tau in the absence (faded) and presence (bold) of heparin.
Similar articles
- O-GlcNAc modification of tau directly inhibits its aggregation without perturbing the conformational properties of tau monomers.
Yuzwa SA, Cheung AH, Okon M, McIntosh LP, Vocadlo DJ. Yuzwa SA, et al. J Mol Biol. 2014 Apr 17;426(8):1736-52. doi: 10.1016/j.jmb.2014.01.004. Epub 2014 Jan 18. J Mol Biol. 2014. PMID: 24444746 - A functional role for intrinsic disorder in the tau-tubulin complex.
Melo AM, Coraor J, Alpha-Cobb G, Elbaum-Garfinkle S, Nath A, Rhoades E. Melo AM, et al. Proc Natl Acad Sci U S A. 2016 Dec 13;113(50):14336-14341. doi: 10.1073/pnas.1610137113. Epub 2016 Nov 23. Proc Natl Acad Sci U S A. 2016. PMID: 27911791 Free PMC article. - Insights into tau function and dysfunction through single-molecule fluorescence.
Melo AM, Elbaum-Garfinkle S, Rhoades E. Melo AM, et al. Methods Cell Biol. 2017;141:27-44. doi: 10.1016/bs.mcb.2017.06.010. Epub 2017 Jul 31. Methods Cell Biol. 2017. PMID: 28882307 Free PMC article. - Amyloidogenesis of Tau protein.
Nizynski B, Dzwolak W, Nieznanski K. Nizynski B, et al. Protein Sci. 2017 Nov;26(11):2126-2150. doi: 10.1002/pro.3275. Epub 2017 Sep 13. Protein Sci. 2017. PMID: 28833749 Free PMC article. Review. - Structural evaluations of tau protein conformation: methodologies and approaches.
Zabik NL, Imhof MM, Martic-Milne S. Zabik NL, et al. Biochem Cell Biol. 2017 Jun;95(3):338-349. doi: 10.1139/bcb-2016-0227. Epub 2017 Mar 9. Biochem Cell Biol. 2017. PMID: 28278386 Free PMC article. Review.
Cited by
- Single Molecule Characterization of Amyloid Oligomers.
Yang J, Perrett S, Wu S. Yang J, et al. Molecules. 2021 Feb 11;26(4):948. doi: 10.3390/molecules26040948. Molecules. 2021. PMID: 33670093 Free PMC article. Review. - Hyperphosphorylation of intrinsically disordered tau protein induces an amyloidogenic shift in its conformational ensemble.
Zhu S, Shala A, Bezginov A, Sljoka A, Audette G, Wilson DJ. Zhu S, et al. PLoS One. 2015 Mar 13;10(3):e0120416. doi: 10.1371/journal.pone.0120416. eCollection 2015. PLoS One. 2015. PMID: 25767879 Free PMC article. - Single-Molecular Förster Resonance Energy Transfer Measurement on Structures and Interactions of Biomolecules.
Qiao Y, Luo Y, Long N, Xing Y, Tu J. Qiao Y, et al. Micromachines (Basel). 2021 Apr 27;12(5):492. doi: 10.3390/mi12050492. Micromachines (Basel). 2021. PMID: 33925350 Free PMC article. Review. - OGlcNAcylation and phosphorylation have opposing structural effects in tau: phosphothreonine induces particular conformational order.
Brister MA, Pandey AK, Bielska AA, Zondlo NJ. Brister MA, et al. J Am Chem Soc. 2014 Mar 12;136(10):3803-16. doi: 10.1021/ja407156m. Epub 2014 Mar 4. J Am Chem Soc. 2014. PMID: 24559475 Free PMC article. - Heparan Sulfate Proteoglycans (HSPGs) Serve as the Mediator Between Monomeric Tau and Its Subsequent Intracellular ERK1/2 Pathway Activation.
Song L, Oseid DE, Wells EA, Coaston T, Robinson AS. Song L, et al. J Mol Neurosci. 2022 Apr;72(4):772-791. doi: 10.1007/s12031-021-01943-2. Epub 2022 Jan 18. J Mol Neurosci. 2022. PMID: 35040015 Free PMC article.
References
- Lee VM, Goedert M, Trojanowski JQ. Annu. Rev. Neurosci. 2001;24:1121. - PubMed
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Brain Res. Rev. 2000;33:95. - PubMed
- Garcia ML, Cleveland DW. Curr. Opin. Cell. Biol. 2001;13:41. - PubMed
- Goedert M, Spillantini MG. Science. 2006;314:777. - PubMed
- Ballatore C, Lee VM, Trojanowski JQ. Na.t Rev. Neuroscience. 2007;8:663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical