Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity - PubMed (original) (raw)
Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity
Sylvain Chauvette et al. Neuron. 2012.
Abstract
Long-term plasticity contributes to memory formation and sleep plays a critical role in memory consolidation. However, it is unclear whether sleep slow oscillation by itself induces long-term plasticity that contributes to memory retention. Using in vivo prethalamic electrical stimulation at 1 Hz, which itself does not induce immediate potentiation of evoked responses, we investigated how the cortical evoked response was modulated by different states of vigilance. We found that somatosensory evoked potentials during wake were enhanced after a slow-wave sleep episode (with or without stimulation during sleep) as compared to a previous wake episode. In vitro, we determined that this enhancement has a postsynaptic mechanism that is calcium dependent, requires hyperpolarization periods (slow waves), and requires a coactivation of both AMPA and NMDA receptors. Our results suggest that long-term potentiation occurs during slow-wave sleep, supporting its contribution to memory.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
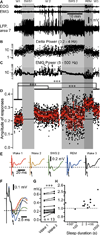
Figure 1. Amplitude of evoked potential responses (N1) to medial lemniscus stimuli throughout sleep-wake periods
(A) Fragment of electro-oculogram (EOG), electromyogram (EMG), and local field potential (LFP) recorded in area 7 of a cat during sleep/wake transitions (W1–3 – Wake; SWS – Slow-wave sleep; REM – Rapid eye movement sleep. Light gray area depicts SWS episodes, darker gray area depicts a REM episode. (B) Dots represent the delta power (area between 0.2 and 4 Hz in the Fast Fourier transform) of 1 second bins from the LFP segment shown in A. (C) Dots represent the EMG power. (D) Black dots represent individual evoked responses (N1) amplitude recorded in the LFP of somatosensory cortex (area 3) to the medial lemniscus stimuli (1Hz). Red squares are the running averages and the standard deviation for 60 responses (1 min). ***p<0.001, one way ANOVA Kruskal-Wallis with Dunn’s multiple comparison test. (E) Averaged (area 3) evoked responses for each states of vigilance. The dotted line here and in panel F indicates the voltage of the mean response amplitude in wake 1, and the arrows point to the component of the response that was studied. (F) Zoom-in and superimposition of averages presented in E (same color code). (G) Group data from 13 “wake1-SWS-wake2” sequences, from 4 cats. Paired comparison of the mean response amplitude during wake1 and wake2 (pre-SWS and post-SWS) episodes. ***p<0.001, Wilcoxon matched-pairs signed rank test. (H) Normalized (to wake 1 mean amplitude of evoked response) change in amplitude of response plotted against sleep duration.
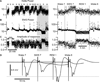
Figure 2. REM sleep does not potentiate somatosensory evoked potential in a following wake episode
The delta power calculated from an area 7 local field potential (A) and EMG power (B) calculated around each medial lemniscal stimulus (±500 ms). (C) The amplitude of somatosensory evoked potential. W – Wake, S – SWS, R – REM sleep. Note that no stimulation was delivered during the first slow-wave sleep episode and that responses were very significantly enhanced in the second wake episode as compared to the first wake episode; ***p<0.001, unpaired t-test with Welch’s correction. The right panel corresponds to the shaded area in (A, B, C) expanded. The amplitude of response was not enhanced after late REM sleep; p=0.7, unpaired t-test with Welch’s correction. (D) Averaged response in each state of vigilance from wake 7 to wake 8 as indicated.
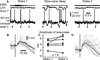
Figure 3. Intracellularly recorded evoked responses are enhanced after a period of slow-wave sleep
(A) Electromyogram (EMG) from neck muscle, surface local field potential (LFP) from area 7, and intracellular recording from somatosensory cortex in consecutive states of vigilance as indicated. Vertical arrows indicate the time of medial lemniscus stimulation. (B, D) Superimposition of 20 individual responses (gray traces) and the averaged response (black trace) during the first (B) and the second (D) episode of wake. Note that responses are ampler in the second episode of wake. (C) Paired comparison of intracellular response amplitude of 6 neurons during two consecutive wake episodes separated by a slow-wave sleep episode. Each symbol represents the averaged response amplitude of one neuron during either wake 1 (left) or wake 2 (right). Lines indicate responses of the same neuron in two conditions.
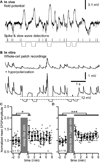
Figure 4. Slow-wave sleep pattern of synaptic stimulation combined to intracellular hyperpolarization pulses induces long-term potentiation in vitro
(A) In vivo field potential recording during a SWS period of a cat. Extracellular spikes were detected and their timing was used for the synaptic stimulation pattern. Slow waves were also detected during the same time period and their timing was used for intracellular membrane potential hyperpolarization. (B) In vitro recordings during sleep-like synaptic stimulation pattern (upper trace) and during the full sleep-like pattern of stimulation (synaptic + hyperpolarizing pulses, lower trace). (C) Group data of normalized EPSP amplitude of in vitro whole-cell recordings in control and after sleep-like synaptic pattern of stimulation. (D) Group data of normalized EPSP amplitude in control and after the full sleep-like pattern of stimulation. Gray dots are individual responses amplitude and black circles are the running averages and standard deviation for 12 consecutive responses (1 min). *p<0.05, ***p<0.001, Mann-Whitney test. Grey boxes indicate the 10 minutes stimulation protocol that was used.
Figure 5. Absence of long-term potentiation after wake pattern of stimulation in vitro
(A) In vivo field potential recording during waking period of a cat. Extracellular spikes were detected and their timing was used as synaptic stimulation pattern. (B) In vitro recordings during wake-like pattern of synaptic stimulation in control (Upper trace) and after adding 200 µM of carbachol (Lower trace). (C) Group data of normalized EPSP amplitude of in vitro whole-cell recordings in control and after wake-like pattern of synaptic stimulation. (D) Group data of normalized EPSP amplitude of in vitro whole-cell recordings in control and after wake-like pattern of synaptic stimulation in presence of carbachol (shaded area). Gray dots are individual responses amplitude and black circles are the running averages and standard deviation for 12 consecutive responses (1 min). *p<0.05, ***p<0.001, Mann-Whitney test. Grey boxes indicate the 10 minutes stimulation protocol that was used.
Figure 6. Properties of long-term plasticity induced by sleep pattern of stimulation
(A) Group data of normalized EPSP amplitude of in vitro whole-cell recordings in control and after shuffled timing of sleep pattern of synaptic stimulation. (B) Group data of normalized EPSP amplitude in control and after a period with the hyperpolarization pattern of stimulation only (no synaptic stimulation). (C) Group data of normalized EPSP amplitude in control and after rhythmic pattern of synaptic stimulation (2.5 Hz). (D) Paired-pulse (ISI 50 ms) test applied before and after the full sleep-like stimulation pattern (synaptic + hyperpolarization). Group data of normalized EPSP amplitude in control and after period with the full sleep-like stimulation pattern in presence of the calcium chelator BAPTA (25 mM) (E), the NMDA antagonist AP5 (100 µM) (F), or the AMPA antagonist CNQX (10 µM) (G). For all panels, gray dots are individual responses amplitude and black circles are the running averages and standard deviation for 12 consecutive responses (1 min). Grey boxes indicate the 10 minutes stimulation protocol that was used.
Comment in
- Sleep to upscale, sleep to downscale: balancing homeostasis and plasticity.
Born J, Feld GB. Born J, et al. Neuron. 2012 Sep 20;75(6):933-5. doi: 10.1016/j.neuron.2012.09.007. Neuron. 2012. PMID: 22998858
Similar articles
- Physiology and pharmacology of corticothalamic stimulation-evoked responses in rat somatosensory thalamic neurons in vitro.
Kao CQ, Coulter DA. Kao CQ, et al. J Neurophysiol. 1997 May;77(5):2661-76. doi: 10.1152/jn.1997.77.5.2661. J Neurophysiol. 1997. PMID: 9163382 - Dynamic Analysis of the Conditional Oscillator Underlying Slow Waves in Thalamocortical Neurons.
David F, Crunelli V, Leresche N, Lambert RC. David F, et al. Front Neural Circuits. 2016 Feb 25;10:10. doi: 10.3389/fncir.2016.00010. eCollection 2016. Front Neural Circuits. 2016. PMID: 26941611 Free PMC article. - Sleep to upscale, sleep to downscale: balancing homeostasis and plasticity.
Born J, Feld GB. Born J, et al. Neuron. 2012 Sep 20;75(6):933-5. doi: 10.1016/j.neuron.2012.09.007. Neuron. 2012. PMID: 22998858 - Neuronal plasticity in thalamocortical networks during sleep and waking oscillations.
Steriade M, Timofeev I. Steriade M, et al. Neuron. 2003 Feb 20;37(4):563-76. doi: 10.1016/s0896-6273(03)00065-5. Neuron. 2003. PMID: 12597855 Review. - Neuronal plasticity and thalamocortical sleep and waking oscillations.
Timofeev I. Timofeev I. Prog Brain Res. 2011;193:121-44. doi: 10.1016/B978-0-444-53839-0.00009-0. Prog Brain Res. 2011. PMID: 21854960 Free PMC article. Review.
Cited by
- Exploring the Evolution of Sleep Patterns From Infancy to Adolescence.
Goel P, Goel A. Goel P, et al. Cureus. 2024 Jul 17;16(7):e64759. doi: 10.7759/cureus.64759. eCollection 2024 Jul. Cureus. 2024. PMID: 39156264 Free PMC article. Review. - Possible mechanisms to improve sleep spindles via closed loop stimulation during slow wave sleep: A computational study.
Mushtaq M, Marshall L, Ul Haq R, Martinetz T. Mushtaq M, et al. PLoS One. 2024 Jun 26;19(6):e0306218. doi: 10.1371/journal.pone.0306218. eCollection 2024. PLoS One. 2024. PMID: 38924001 Free PMC article. - Sleep pressure modulates single-neuron synapse number in zebrafish.
Suppermpool A, Lyons DG, Broom E, Rihel J. Suppermpool A, et al. Nature. 2024 May;629(8012):639-645. doi: 10.1038/s41586-024-07367-3. Epub 2024 May 1. Nature. 2024. PMID: 38693264 Free PMC article. - Human REM sleep recalibrates neural activity in support of memory formation.
Lendner JD, Niethard N, Mander BA, van Schalkwijk FJ, Schuh-Hofer S, Schmidt H, Knight RT, Born J, Walker MP, Lin JJ, Helfrich RF. Lendner JD, et al. Sci Adv. 2023 Aug 25;9(34):eadj1895. doi: 10.1126/sciadv.adj1895. Epub 2023 Aug 25. Sci Adv. 2023. PMID: 37624898 Free PMC article. - GABAergic synaptic transmission and plasticity oscillate across sleep and wake.
Wu K, Lu W. Wu K, et al. Neural Regen Res. 2023 Dec;18(12):2647-2648. doi: 10.4103/1673-5374.373665. Neural Regen Res. 2023. PMID: 37449604 Free PMC article. No abstract available.
References
- Alloway KD, Wallace MB, Johnson MJ. Cross-correlation analysis of cuneothalamic interactions in the rat somatosensory system: influence of receptive field topography and comparisons with thalamocortical interactions. J Neurophysiol. 1994;72:1949–1972. - PubMed
- Chang HT. Cortical response to activity of callosal neurons. J Neurophysiol. 1953;16:117–131. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS060870/NS/NINDS NIH HHS/United States
- MOP-67175/CAPMC/ CIHR/Canada
- 1R01NS060870/NS/NINDS NIH HHS/United States
- MOP-37862/CAPMC/ CIHR/Canada
- R01 NS059740/NS/NINDS NIH HHS/United States
- 1R01NS059740/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources