Targeting the TGFβ signalling pathway in disease - PubMed (original) (raw)
Review
. 2012 Oct;11(10):790-811.
doi: 10.1038/nrd3810. Epub 2012 Sep 24.
Affiliations
- PMID: 23000686
- PMCID: PMC3520610
- DOI: 10.1038/nrd3810
Review
Targeting the TGFβ signalling pathway in disease
Rosemary J Akhurst et al. Nat Rev Drug Discov. 2012 Oct.
Abstract
Many drugs that target transforming growth factor-β (TGFβ) signalling have been developed, some of which have reached Phase III clinical trials for a number of disease applications. Preclinical and clinical studies indicate the utility of these agents in fibrosis and oncology, particularly in augmentation of existing cancer therapies, such as radiation and chemotherapy, as well as in tumour vaccines. There are also reports of specialized applications, such as the reduction of vascular symptoms of Marfan syndrome. Here, we consider why the TGFβ signalling pathway is a drug target, the potential clinical applications of TGFβ inhibition, the issues arising with anti-TGFβ therapy and how these might be tackled using personalized approaches to dosing, monitoring of biomarkers as well as brief and/or localized drug-dosing regimens.
Figures
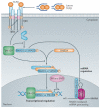
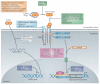
Figure 1. Schematic overview of the canonical, SMAD-dependent TGFβ signalling pathway
The transforming growth factor-β (TGFβ) ligands are synthesized as a large latent TGFβ complex consisting of mature dimeric TGFβ associated with its latency-associated peptide (LAP) and a latent TGFβ-binding protein (LTBP) (not shown). Upon activation, TGFβ dimers induce heteromeric complex formation between specific type II and type I receptors (such as TGFβ receptortype II (TpRII) andTpRI, respectively). Type II receptors then transphosphorylate the type I receptors, which propagate the signal into the cell by phosphorylating TGFβ receptor-specific SMADs (R-SMADs: SMAD2 and SMAD3). They form heteromeric complexes with the common mediator SMAD (co-SMAD: SMAD4) and translocate to the nucleus. Once in the nucleus, the R-SMAD-co-SMAD complex preferentially associates with the genomic SMAD-binding element (SBE) in a sequence-specific manner. However, high-affinity binding of the R-SMAD-co-SMAD complex with the SBE generally occurs in concert with other DNA-binding transcription factors that bind to distinct sequences adjacent to the SBE. The nuclear proteins SKI and SNO (also known as SKIL) antagonize the transcriptional regulation by SMADs. An inhibitory SMAD (I-SMAD), SMAD7, inhibits the TGFβ pathway through multiple mechanisms, including by mediating the degradation of the type I receptor, inhibiting phosphorylation of R-SMADs by the type I receptor kinase or inhibiting the formation of the R-SMAD–co-SMAD complex. In addition to regulating transcription, R-SMADs can modulate microRNA (miRNA) biogenesis by facilitating the processing of primary miRNA into precursor miRNA in the nucleus. The co-SMAD is not required for the regulation of miRNA biosynthesis by R-SMADs. ‘mG’ and ‘AAAAA’ represent 5′ capping and 3′ polyadenylation of mRNAs, respectively.
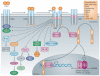
Figure 2. Schematic representation of non-canonical TGFβ signalling and crosstalk with other signalling pathways
In the non-canonical pathways, the activated transforming growth factor-β (TGFβ) receptor complex transmits a signal through other factors, such as TNF receptor associated factor 4 (TRAF4) or TRAF6, TGFβ-activated kinase 1 (TAK1), p38 mitogen-activated protein kinase (p38 MAPK), RHO, phosphoinositide 3-kinase (PI3K)-AKT, extracellular signal-regulated kinase (ERK), JUN N-terminal kinase (JNK) or nuclear factor-KB (NF-kB). TGFβ signalling can be influenced by pathways other than the canonical and non-canonical TGFβ signalling pathways, such as the WNT, Hedgehog, Notch, interferon (IFN), tumour necrosis factor (TNF) and RAS pathways. The crosstalk between TGFβ and other pathways defines the activities of TGFβ to propagate spatial- and temporal-specific signals. miRNA, microRNA; ROCK, RHO-associated protein kinase; R-SMAD, receptor-specific SMAD; TpR, TGFβ receptor. ‘mG’ and ‘AAAAA’ represent 5′ capping and 3′ polyadenylation of mRNAs, respectively.
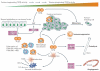
Figure 3. Biphasic activities of the TGFβ signalling pathway during tumorigenesis: from the tumour suppressor to the tumour promoter
Transforming growth factor-β (TGFβ) has biphasic actions during tumorig enesis, suppressing tumorigenesis at early stages but promoting tumour progression later on, which is the underlying paradigm for the action of TGFβ during disease progression in general and thus complicates the development of therapies targeting TGFβ signalling. The light grey arrows indicate a positive feedforward loop resulting in higher levels of TGFβ, which is a feature of non-neoplastic as well as neoplastic diseases. The current goal in cancer therapy is to downmodulate excessive levels of TGFβ ligands and to target the tumour-progressing versus the tumour-suppressing arm of TGFβ action; the latter goal will almost certainly require more-specific second-generation drugs. CTGF, connective tissue growth factor; EMT, epithelial-mesenchymal transition; IL, interleukin; PTHRP, parathyroid hormone-related protein; TAMs, tumour-associated macrophages; TANs, tumour-associated neutrophils; VEGF, vascular endothelial growth factor.
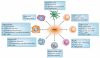
Figure 4. TGFβ effects on immune cells
Transforming growth factor-p (TGFβ) has effects on most immune cell types. The figure depicts the activity of TGFβ on immune cell subsets that is relevant to human diseases. M1→M2 and N1→N2 indicate polarization of macrophages and neutrophils, respectively, from type I to type II. IgA, immunoglobulin A; TH, T helper; TReg, regulatory T.
Figure 5. Schematic representation of therapeutic approaches for blocking TGFβ signalling
Transforming growth factor-p (TGFβ) signalling can be inhibited by: sequestering ligands using soluble receptor ectodomain constructs (ligand traps) derived from TGFβ receptortype II (TpRII) or TpRIII; using TGFβ-neutralizing antibodies; or with TpRII or TpRI kinase inhibitors. Furthermore, translation of TGFβ mRNA can be blocked by targeting TGFβ mRNA with antisense oligonucleotides, thus preventing the production of the ligand. Different small-molecule kinase inhibitors against TpRI have been developed to block its kinase activity. Peptide inhibitors against specific TGFβ ligands are also used. Other approaches block the transformation of TGFβ from the latent to the active form. Three molecules are shown that either affect TGFβ signalling indirectly (losartan) or that have an as-yet-unidentified target (tranilast and pirfenidone). All of these approaches decrease the initiation of intracellular receptor signalling pathways, such as phosphorylation of downstream receptor-specific SMADs (R-SMADs), and thereby blunt the transcriptional regulation of target genes. ATI, angiotensin II type 1 receptor; co-TFs, co-transcription factors; FOXH1B, forkhead box protein H1B; LEF, lymphoid enhancer-binding factor; LSKL, Leu-Ser-Lys-Leu peptide; TRX, thioredoxin.
Figure 6. Structures of representative small-molecule inhibitors of TGFβ signalling
Depicted are the molecular structures of a selection of small-molecule inhibitors identified to target the transforming growth factor-p (TGFβ) signalling pathway. SB-431542, LY2157299, SD208 and SM16 are all ATP mimetics that inhibit TGFβ receptortype I (TpRI; also known asTGFBRl) kinase activity. Pyrrole-imidazole polyamide blocks transcription of the TGFB1 gene. Pirfenidone and tranilast have unknown molecular mechanisms of action. Dashed lines denote putative hydrogen bonding with bases in DNA; asterisks indicate positions where hydrogen bonds form with nucleotide residues of DNA within the TGFB1 gene promoter.
Similar articles
- Targeting the TGFβ pathway for cancer therapy.
Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, de Gramont A. Neuzillet C, et al. Pharmacol Ther. 2015 Mar;147:22-31. doi: 10.1016/j.pharmthera.2014.11.001. Epub 2014 Nov 6. Pharmacol Ther. 2015. PMID: 25444759 Review. - The TGFBeta pathway as a therapeutic target in cancer.
Seoane J. Seoane J. Clin Transl Oncol. 2008 Jan;10(1):14-9. doi: 10.1007/s12094-008-0148-2. Clin Transl Oncol. 2008. PMID: 18208788 Review. - Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFβ signalling.
Beyer C, Zenzmaier C, Palumbo-Zerr K, Mancuso R, Distler A, Dees C, Zerr P, Huang J, Maier C, Pachowsky ML, Friebe A, Sandner P, Distler O, Schett G, Berger P, Distler JH. Beyer C, et al. Ann Rheum Dis. 2015 Jul;74(7):1408-16. doi: 10.1136/annrheumdis-2013-204508. Epub 2014 Feb 23. Ann Rheum Dis. 2015. PMID: 24567525 - Large- and small-molecule inhibitors of transforming growth factor-beta signaling.
Akhurst RJ. Akhurst RJ. Curr Opin Investig Drugs. 2006 Jun;7(6):513-21. Curr Opin Investig Drugs. 2006. PMID: 16784021 Review. - Targeting TGFbeta-mediated processes in cancer.
Romano MF. Romano MF. Curr Opin Drug Discov Devel. 2009 Mar;12(2):253-63. Curr Opin Drug Discov Devel. 2009. PMID: 19333871 Review.
Cited by
- Systems analysis of a RIG-I agonist inducing broad spectrum inhibition of virus infectivity.
Goulet ML, Olagnier D, Xu Z, Paz S, Belgnaoui SM, Lafferty EI, Janelle V, Arguello M, Paquet M, Ghneim K, Richards S, Smith A, Wilkinson P, Cameron M, Kalinke U, Qureshi S, Lamarre A, Haddad EK, Sekaly RP, Peri S, Balachandran S, Lin R, Hiscott J. Goulet ML, et al. PLoS Pathog. 2013;9(4):e1003298. doi: 10.1371/journal.ppat.1003298. Epub 2013 Apr 25. PLoS Pathog. 2013. PMID: 23633948 Free PMC article. - MicroRNA regulation of macrophages in human pathologies.
Wei Y, Schober A. Wei Y, et al. Cell Mol Life Sci. 2016 Sep;73(18):3473-95. doi: 10.1007/s00018-016-2254-6. Epub 2016 May 2. Cell Mol Life Sci. 2016. PMID: 27137182 Free PMC article. Review. - Adipocytes fail to maintain cellular identity during obesity due to reduced PPARγ activity and elevated TGFβ-SMAD signaling.
Roh HC, Kumari M, Taleb S, Tenen D, Jacobs C, Lyubetskaya A, Tsai LT, Rosen ED. Roh HC, et al. Mol Metab. 2020 Dec;42:101086. doi: 10.1016/j.molmet.2020.101086. Epub 2020 Sep 28. Mol Metab. 2020. PMID: 32992037 Free PMC article. - Skeletal muscle regeneration via the chemical induction and expansion of myogenic stem cells in situ or in vitro.
Fang J, Sia J, Soto J, Wang P, Li LK, Hsueh YY, Sun R, Faull KF, Tidball JG, Li S. Fang J, et al. Nat Biomed Eng. 2021 Aug;5(8):864-879. doi: 10.1038/s41551-021-00696-y. Epub 2021 Mar 18. Nat Biomed Eng. 2021. PMID: 33737730 Free PMC article. - Force Matters: Biomechanical Regulation of Cell Invasion and Migration in Disease.
Kai F, Laklai H, Weaver VM. Kai F, et al. Trends Cell Biol. 2016 Jul;26(7):486-497. doi: 10.1016/j.tcb.2016.03.007. Epub 2016 Apr 4. Trends Cell Biol. 2016. PMID: 27056543 Free PMC article. Review.
References
- Schmierer B, Hill CS. TGFβ-SMAD signal transduction: molecular specificity and functional flexibility. Nature Rev. Mol. Cell Biol. 2007;8:970–982. - PubMed
- Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-β family proteins in development and disease. Nature Cell Biol. 2007;9:1000–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA116019/CA/NCI NIH HHS/United States
- R01 GM060514/GM/NIGMS NIH HHS/United States
- R01 HL078564/HL/NHLBI NIH HHS/United States
- P30 CA082103/CA/NCI NIH HHS/United States
- P01 AR050440/AR/NIAMS NIH HHS/United States
- R01 HL108317/HL/NHLBI NIH HHS/United States
- R21 CA164772/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources