Effect of maternal obesity on estrous cyclicity, embryo development and blastocyst gene expression in a mouse model - PubMed (original) (raw)
Effect of maternal obesity on estrous cyclicity, embryo development and blastocyst gene expression in a mouse model
Pablo Bermejo-Alvarez et al. Hum Reprod. 2012 Dec.
Abstract
Study question: Does maternal obesity affect estrous cyclicity, embryo development and blastocyst gene expression in mice?
Summary answer: Maternal obesity alters estrous cyclicity and causes the down-regulation of two key metabolite receptors (Slc2a1 and Ldlr) in blastocysts recovered from diet-induced obese females, but embryo development is not affected.
What is known already: Maternal obesity reduces fertility because of effects in the periconception period, but its negative influence is on estrous cyclicity, oocyte quality or embryo development.
Study design, size and duration: This was a randomized study based on a mouse model for obesity. Twenty-one outbred NIH Swiss mice were used and obesity was induced by a diet high in fat administered for 12 weeks prior to breeding to control males.
Material, setting and methods: Females were fed either a control diet (C, n = 9) or a diet high in fat [diet-induced obesity (DiO), n = 12] for 12 weeks, and were then co-housed with fertile males. Mice that failed to breed during 20 consecutive days were considered infertile. Control and diet-induced obese females that demonstrated vaginal plugs were euthanized 3.5 days after mating, blood was sampled for glucose and hormone measurements, corpora lutea counted and embryos recovered; the relative mRNA abundance of 11 candidate genes was determined in blastocysts by qPCR.
Main results and the role of chance: Five DiO females failed to breed and displayed anovulatory ovaries (DiOI), whereas the other seven DiO females (DiOF) could breed, albeit over an extended period compared with controls. DiOF weighed significantly less than DiOI. Both groups had elevated serum insulin compared with C, although blood glucose level was only significantly higher than that in controls in the infertile DiOI group. Adiponectin was lower in the DiOI and leptin higher in both the DiOI and DiOF mice than in C. DiOF ovulated the same number of oocytes as C, and embryo development to blastocyst was normal. The expression of genes encoding metabolic hormone receptors (Insr, Igf1r, Igf2r, Adipor1, Adipor2 and Lepr) and key metabolic enzymes (Gapdh, Cpt1a and Sod2) did not differ between DiOF and C blastocysts, but that of metabolite receptors (Slc2a1 and Ldr) was down-regulated in DiOF. To limit the role of chance, the experiments were conducted in a defined laboratory setting with the proper controls, and the animals were randomly assigned to each experimental group. Moreover, a P-value of < 0.05 was chosen to determine whether the differences observed between the groups were statistically significant.
Limitations and reasons for caution: The results obtained may not fully extrapolate to humans. Also, as follicular activity was not monitored while breeding, so the extended breeding period for DiOF group might be explained by behavioral abnormalities occurring in normal cycling animals.
Wider implications of the findings: DiO alters the estrous cycle in the mouse model and demonstrates a role of obesity in infertility. The data also suggest that in an outbred, genetically diverse population, such as the human, individual susceptibility to obesity and associated infertility induced by diet exists. The apparently normal development to blastocyst observed in fertile, obese females suggests that preimplantation embryos can resist potentially adverse outcomes caused by an oversupply of fatty acids and glucose under in vivo conditions. This metabolic plasticity may, in part, be due to an ability to down-regulate metabolite transporters, thereby preventing excessive nutrient uptake.
Study funding/competing interest(s): The research was supported by funds from the University of Missouri, grants from the National Institutes of Health and by a fellowship from the Lalor Foundation. There were no competing interests.
Trial registration number: Not applicable.
Figures
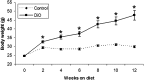
Figure 1
Body weight divergence of mice on the control and high-fat (DiO) diets over 12 weeks (mean ± SEM). Asterisks denote significant differences between control and DiO females based on ANOVA (P < 0.05).
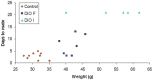
Figure 2
Relationship between days required to observe a copulatory plug and body weight for mice maintained on the control and DiO diets for 12 weeks. Individual data are for Control (orange diamonds, n = 9), fertile obese (DiOF, dark blue squares, n = 7) and infertile obese females that failed to breed over a 20-day period (DiOI, light blue triangles, n = 5).
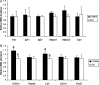
Figure 3
Relative mRNA abundance for candidate genes expressed in blastocysts recovered from Control and DiOF dams. Data (mean ± SEM) are for control (black, n = 9) and DiO (white, n = 7). (A) Values for genes encoding for metabolic hormone; (B) genes implicated in metabolite transport (Slc2a1 and Ldlr), key metabolic pathways (Gapdh and Ldlr) and ROS detoxification (Sod2). Different letters indicate significant differences between groups based on ANOVA (P < 0.05).
Similar articles
- Combined parental obesity negatively impacts preimplantation mouse embryo development, kinetics, morphology and metabolism.
Finger BJ, Harvey AJ, Green MP, Gardner DK. Finger BJ, et al. Hum Reprod. 2015 Sep;30(9):2084-96. doi: 10.1093/humrep/dev142. Epub 2015 Jun 18. Hum Reprod. 2015. PMID: 26089300 - Preimplantation maternal stress impairs embryo development by inducing oviductal apoptosis with activation of the Fas system.
Zheng LL, Tan XW, Cui XZ, Yuan HJ, Li H, Jiao GZ, Ji CL, Tan JH. Zheng LL, et al. Mol Hum Reprod. 2016 Nov;22(11):778-790. doi: 10.1093/molehr/gaw052. Epub 2016 Jul 29. Mol Hum Reprod. 2016. PMID: 27475493 - Germline nuclear transfer in mice may rescue poor embryo development associated with advanced maternal age and early embryo arrest.
Tang M, Popovic M, Stamatiadis P, Van der Jeught M, Van Coster R, Deforce D, De Sutter P, Coucke P, Menten B, Stoop D, Boel A, Heindryckx B. Tang M, et al. Hum Reprod. 2020 Jul 1;35(7):1562-1577. doi: 10.1093/humrep/deaa112. Hum Reprod. 2020. PMID: 32613230 - Do little embryos make big decisions? How maternal dietary protein restriction can permanently change an embryo's potential, affecting adult health.
Fleming TP, Watkins AJ, Sun C, Velazquez MA, Smyth NR, Eckert JJ. Fleming TP, et al. Reprod Fertil Dev. 2015 May;27(4):684-92. doi: 10.1071/RD14455. Reprod Fertil Dev. 2015. PMID: 25730413 Review. - The subcortical maternal complex: emerging roles and novel perspectives.
Bebbere D, Albertini DF, Coticchio G, Borini A, Ledda S. Bebbere D, et al. Mol Hum Reprod. 2021 Jul 1;27(7):gaab043. doi: 10.1093/molehr/gaab043. Mol Hum Reprod. 2021. PMID: 34191027 Review.
Cited by
- Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics.
Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Gabory A, et al. Biol Sex Differ. 2013 Mar 21;4(1):5. doi: 10.1186/2042-6410-4-5. Biol Sex Differ. 2013. PMID: 23514128 Free PMC article. - Effect of lipotoxicity on mitochondrial function and epigenetic programming during bovine in vitro embryo production.
Meulders B, Marei WFA, Xhonneux I, Bols PEJ, Leroy JLMR. Meulders B, et al. Sci Rep. 2023 Dec 8;13(1):21664. doi: 10.1038/s41598-023-49184-0. Sci Rep. 2023. PMID: 38066095 Free PMC article. - Exposure to maternal obesity alters gene expression in the preimplantation ovine conceptus.
McCoski SR, Vailes MT, Owens CE, Cockrum RR, Ealy AD. McCoski SR, et al. BMC Genomics. 2018 Oct 11;19(1):737. doi: 10.1186/s12864-018-5120-0. BMC Genomics. 2018. PMID: 30305020 Free PMC article. - Single-cell RNA sequencing reveals the effects of high-fat diet on oocyte and early embryo development in female mice.
Zhu Q, Li F, Wang H, Wang X, Xiang Y, Ding H, Wu H, Xu C, Weng L, Cai J, Xu T, Liang N, Hong X, Xue M, Ge H. Zhu Q, et al. Reprod Biol Endocrinol. 2024 Aug 20;22(1):105. doi: 10.1186/s12958-024-01279-7. Reprod Biol Endocrinol. 2024. PMID: 39164729 Free PMC article. - Treatment with a dual amylin and calcitonin receptor agonist improves metabolic health in an old, obese, and ovariectomized rat model.
Katri A, Reker D, Karsdal MA, Bay-Jensen AC, Henriksen K. Katri A, et al. Menopause. 2021 Jan 4;28(4):423-430. doi: 10.1097/GME.0000000000001722. Menopause. 2021. PMID: 33399320 Free PMC article.
References
- Agarwal SK, Vogel K, Weitsman SR, Magoffin DA. Leptin antagonizes the insulin-like growth factor-I augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J Clin Endocrinol Metab. 1999;84:1072–1076. - PubMed
- Akamine EH, Marcal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, Carvalho CR. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. 2010;206:65–74. - PubMed
- Alexenko AP, Mao J, Ellersieck MR, Davis AM, Whyte JJ, Rosenfeld CS, Roberts RM. The contrasting effects of ad libitum and restricted feeding of a diet very high in saturated fats on sex ratio and metabolic hormones in mice. Biol Reprod. 2007;77:599–604. - PubMed
- Arias-Alvarez M, Bermejo-Alvarez P, Gutierrez-Adan A, Rizos D, Lorenzo PL, Lonergan P. Effect of leptin supplementation during in vitro oocyte maturation and embryo culture on bovine embryo development and gene expression patterns. Theriogenology. 2011;75:887–896. - PubMed
- Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, West C, Jacobs HS. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod. 1995;10:2107–2111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous