Genetic and biochemical characterizations of enzymes involved in Streptococcus pneumoniae serotype 2 capsule synthesis demonstrate that Cps2T (WchF) catalyzes the committed step by addition of β1-4 rhamnose, the second sugar residue in the repeat unit - PubMed (original) (raw)
Genetic and biochemical characterizations of enzymes involved in Streptococcus pneumoniae serotype 2 capsule synthesis demonstrate that Cps2T (WchF) catalyzes the committed step by addition of β1-4 rhamnose, the second sugar residue in the repeat unit
David B A James et al. J Bacteriol. 2012 Dec.
Abstract
Five genes (cps2E, cps2T, cps2F, cps2G, and cps2I) are predicted to encode the glycosyltransferases responsible for synthesis of the Streptococcus pneumoniae serotype 2 capsule repeat unit, which is polymerized to yield a branched surface structure containing glucose-glucuronic acid linked to a glucose-rhamnose-rhamnose-rhamnose backbone. Cps2E is the initiating glycosyltransferase, but experimental evidence supporting the functions of the remaining glycosyltransferases is lacking. To biochemically characterize the glycosyltransferases, the donor substrate dTDP-rhamnose was first synthesized using recombinant S. pneumoniae enzymes Cps2L, Cps2M, Cps2N, and Cps2O. In in vitro assays with each of the glycosyltransferases, only reaction mixtures containing recombinant Cps2T, dTDP-rhamnose, and the Cps2E product (undecaprenyl pyrophosphate glucose) generated a new product, which was consistent with lipid-linked glucose-rhamnose. cps2T, cps2F, and cps2I deletion mutants produced no detectable capsule, but trace amounts of capsule were detectable in Δcps2G mutants, suggesting that Cps2G adds a nonbackbone sugar. All Δcps2F, Δcps2G, and Δcps2I mutants contained different secondary suppressor mutations in cps2E, indicating that the initial mutations were lethal in the absence of reduced repeat unit synthesis. Δcps2T mutants did not contain secondary mutations affecting capsule synthesis. The requirement for secondary mutations in mutants lacking Cps2F, Cps2G, and Cps2I indicates that these activities occur downstream of the committed step in capsule synthesis and reveal that Cps2T catalyzes this step. Therefore, Cps2T is the β1-4 rhamnosyltransferase that adds the second sugar to the repeat unit and, as the committed step in type 2 repeat unit synthesis, is predicted to be an important point of capsule regulation.
Figures
Fig 1
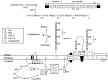
S. pneumoniae type 2 genetic locus, capsular polysaccharide repeat unit structure, and Wzy-dependent pathway. (Top) cps2 genetic locus. The arrow indicates the direction of transcription and the predicted operon. (Middle) Repeat unit structure. Letters below each glycosidic linkage represent the glycosyltransferase known or predicted to catalyze the linkage. Predicted enzymes are in parentheses and are based on previous work (1), where the specificities of Cps2G and Cps2I were unclear. Glc, glucose; Rha, rhamnose; GlcUA, glucuronic acid. E, Cps2E; T, Cps2T; F, Cps2F; G, Cps2G; I, Cps2I. (Bottom) Wzy-dependent model for synthesis of the type 2 repeat unit. Synthesis of the capsule repeat unit occurs through the activity of multiple glycosyltransferases. The completed repeat unit is transferred to the outer face of the cytoplasmic membrane, is polymerized, and then remains associated with the membrane or is released or attached to peptidoglycan. With the exception of GalU (the UDP-glucose pyrophosphorylase encoded by galU), all gene products are encoded in the cps2 locus. (Adapted from reference .)
Fig 2
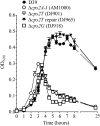
Growth of D39 and derivatives. Cultures grown in THY to an optical density at 600 nm (OD600) of 0.15 were diluted 1/10 and grown in THY. _A_600 readings were taken at the indicated time points. Data are the means and standard errors from two cultures. AM1000 is a capsule-negative mutant of D39.
Fig 3
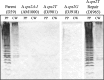
Capsule production by D39 and derivatives. Protoplast (membrane-containing) and cell wall fractions were extracted as described in Materials and Methods. Samples were separated by SDS–12% PAGE and transferred to a nitrocellulose membrane. Capsule was detected using polyclonal antiserum against the type 2 capsule. Protoplasts (PP) were normalized to 30 μg of total protein, and cell wall (CW) fractions were loaded relative to their protoplast counterparts. AM1000 is a capsule-negative mutant of D39.
Fig 4
dTDP-Rha biosynthesis. (A) dTDP-Rha biosynthesis pathway in S. pneumoniae type 2. (B) dTDP-Rha biosynthesis enzymes were expressed in E. coli and purified using an incorporated N-terminal His tag. Purified proteins (0.1 μg) were separated by SDS–12% PAGE and stained with Coomassie brilliant blue. Lanes: 1, Cps2L (calculated molecular mass, 34.7 kDa); 2, Cps2N (41.5 kDa); 3, Cps2M (24.8 kDa); 4, Cps2O (34.8 kDa). Apparent molecular masses from standards are presented on the left. (C) HPLC analyses of the Cps2L, Cps2N, Cps2M, and Cps2O reaction products. Samples analyzed, listed from the top to bottom, are dTDP-Glc standard, Cps2L, Cps2N, Cps2M, and Cps2O reaction products. (D) GC-MS sugar component analyses of Glc standard, Rha standard, and purified Cps2O product.
Fig 5
Cps2T, Cps2F, Cps2G, and Cps2I localization in E. coli. (A) N-terminal His-tagged glycosyltransferases were expressed in E. coli. The cytoplasmic and membrane fractions were extracted and normalized as described in Materials and Methods. Three micrograms of total protein for each fraction was separated by SDS–12% PAGE, transferred to a nitrocellulose membrane, and probed with anti-tetra-His antibody. The calculated molecular masses for His-tagged Cps2T, Cps2F, Cps2G, and Cps2I are 47.6, 38.7, 42.9, and 46.8 kDa, respectively. (B) Fractionation controls, using representative preparations from DJ052 (Cps2T), were probed with MAb to the β subunit of E. coli RNA polymerase (150 kDa) for cytoplasmic protein detection (left) and with MAb to the β subunit of ATP synthase (50 kDa) for membrane protein detection (right). C, cytoplasmic fraction; M, membrane fraction.
Fig 6
Cps2T rhamnosyltransferase activity. Cps2E-containing membranes (3 μg total membrane protein) were incubated with membranes (3 μg total protein) containing the indicated non-His-tagged glycosyltransferases or membrane controls (MC; 3 μg total protein from vector-only E. coli strain RC124) and UDP-[14C]Glc only (A) or UDP-[14C]Glc and dTDP-Rha (B). Reaction mixtures (total volume, 75 μl) were incubated for 1 h at 10°C and contained 0.008% NP-40, 10 mM MnCl2, 1 mM DTT, 0.025 μCi UDP[14C]Glc (293 mCi/mmol), and, for panels B and C, 0.1 mM dTDP-Rha. Glycolipids were extracted, mild-acid hydrolyzed to liberate the saccharide moiety, and separated by TLC as described in Materials and Methods. The migration of the product designated Glc was equivalent to that of a [14C]Glc standard. (C and D) Cps2T activity in the absence of Cps2E. (C) The extracted Cps2E glycolipid product (Und-P-P-[14C]Glc) was incubated with membranes containing non-His-tagged Cps2T or membranes from the vector-only E. coli strain (MC). Reaction mixtures contained 3 μg of total membrane protein, 0.008% NP-40, 10 mM MnCl2, 1 mM DTT, 25 μl of the Und-P-P-[14C]Glc solution (see Materials and Methods), and 0.1 mM dTDP-Rha. Reactions were processed as described above. (D) Cps2E immunoblot demonstrating controls for extracted glycolipids. Lanes: 1, 3 μg of total membrane protein before extraction; 2, extracted organic phase containing glycolipids; 3, interface between organic and aqueous phase containing extracted proteins.
Similar articles
- Biochemical activities of Streptococcus pneumoniae serotype 2 capsular glycosyltransferases and significance of suppressor mutations affecting the initiating glycosyltransferase Cps2E.
James DB, Gupta K, Hauser JR, Yother J. James DB, et al. J Bacteriol. 2013 Dec;195(24):5469-78. doi: 10.1128/JB.00715-13. Epub 2013 Oct 4. J Bacteriol. 2013. PMID: 24097952 Free PMC article. - CpsE from type 2 Streptococcus pneumoniae catalyzes the reversible addition of glucose-1-phosphate to a polyprenyl phosphate acceptor, initiating type 2 capsule repeat unit formation.
Cartee RT, Forsee WT, Bender MH, Ambrose KD, Yother J. Cartee RT, et al. J Bacteriol. 2005 Nov;187(21):7425-33. doi: 10.1128/JB.187.21.7425-7433.2005. J Bacteriol. 2005. PMID: 16237026 Free PMC article. - The metabolism of 6-deoxyhexoses in bacterial and animal cells.
Tonetti M, Sturla L, Bisso A, Zanardi D, Benatti U, De Flora A. Tonetti M, et al. Biochimie. 1998 Nov;80(11):923-31. doi: 10.1016/s0300-9084(00)88889-6. Biochimie. 1998. PMID: 9893952 Review. - Bacterial capsular polysaccharide and sugar transferases.
Miyake K, Iijima S. Miyake K, et al. Adv Biochem Eng Biotechnol. 2004;90:89-111. doi: 10.1007/b94193. Adv Biochem Eng Biotechnol. 2004. PMID: 15453186 Review.
Cited by
- Rhamnose-Containing Compounds: Biosynthesis and Applications.
Li S, Chen F, Li Y, Wang L, Li H, Gu G, Li E. Li S, et al. Molecules. 2022 Aug 20;27(16):5315. doi: 10.3390/molecules27165315. Molecules. 2022. PMID: 36014553 Free PMC article. Review. - Biochemical activities of Streptococcus pneumoniae serotype 2 capsular glycosyltransferases and significance of suppressor mutations affecting the initiating glycosyltransferase Cps2E.
James DB, Gupta K, Hauser JR, Yother J. James DB, et al. J Bacteriol. 2013 Dec;195(24):5469-78. doi: 10.1128/JB.00715-13. Epub 2013 Oct 4. J Bacteriol. 2013. PMID: 24097952 Free PMC article. - Functional identification of a galactosyltransferase critical to Bacteroides fragilis Capsular Polysaccharide A biosynthesis.
Troutman JM, Sharma S, Erickson KM, Martinez CD. Troutman JM, et al. Carbohydr Res. 2014 Aug 18;395:19-28. doi: 10.1016/j.carres.2014.06.003. Epub 2014 Jun 14. Carbohydr Res. 2014. PMID: 24997288 Free PMC article. - Streptococcus mutans requires mature rhamnose-glucose polysaccharides for proper pathophysiology, morphogenesis and cellular division.
Kovacs CJ, Faustoferri RC, Bischer AP, Quivey RG Jr. Kovacs CJ, et al. Mol Microbiol. 2019 Sep;112(3):944-959. doi: 10.1111/mmi.14330. Epub 2019 Jul 12. Mol Microbiol. 2019. PMID: 31210392 Free PMC article. - Population-based analysis of invasive nontypeable pneumococci reveals that most have defective capsule synthesis genes.
Park IH, Geno KA, Sherwood LK, Nahm MH, Beall B. Park IH, et al. PLoS One. 2014 May 15;9(5):e97825. doi: 10.1371/journal.pone.0097825. eCollection 2014. PLoS One. 2014. PMID: 24831650 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30A127767/PHS HHS/United States
- AI28457/AI/NIAID NIH HHS/United States
- P50 AT00477/AT/NCCIH NIH HHS/United States
- P50 AT000477/AT/NCCIH NIH HHS/United States
- U54 CA 100949/CA/NCI NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- R01 AI028457/AI/NIAID NIH HHS/United States
- P30 AR50948/AR/NIAMS NIH HHS/United States
- U54 CA100949/CA/NCI NIH HHS/United States
- P30 AR050948/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases