Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice - PubMed (original) (raw)
Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice
Csaba Varga et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Endogenous brain rhythms occurring at various frequencies and associated with distinct behavioral states provide multiscale temporal windows that enable cells to time their spiking activity with high precision, which is thought to be important for the coding of information in neuronal circuits. However, although the selective timing of GABAergic inputs to specific spatial domains of principal cells are known to play key roles in network oscillations, the in vivo firing patterns of distinct hippocampal interneurons in awake animals are not known. Here we used a combination of juxtacellular labeling techniques with recordings from anesthesia-free, head-fixed mice running or resting on a spherical treadmill to study the oscillation-dependent discharges by two major interneuronal subtypes, the perisomatically projecting parvalbumin-positive basket cells (PVBCs) and distal dendritically projecting oriens lacunosum moleculare (OLM) cells. Recordings of the spiking activity of post hoc-identified CA1 interneurons during theta (5-10 Hz), gamma (25-90Hz), epsilon ("high-gamma"; 90-130 Hz), and ripple (130-200 Hz) oscillations revealed both cell type- and behavioral state-dependent entrainments of PVBC and OLM cell discharges in awake mice. Our results in awake mice differed in several respects from previous data on interneuronal discharge patterns in anesthetized animals. In addition, our results demonstrate a form of frequency-invariant, cell type-specific temporal ordering of inhibitory inputs in which PVBC-derived perisomatic inhibition is followed by OLM cell-generated distal dendritic inhibition during each of the network oscillation bands studied, spanning more than an order of magnitude in frequencies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
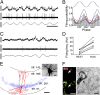
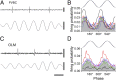
Entrainment of PVBCs during theta oscillations in anesthesia-free mice. (A) Single-unit activity of a PVBC during running-associated theta oscillations. Filter settings for the illustrated traces: (Top) 0.8 Hz–5 kHz; (Middle) 500 Hz high-pass; (Bottom) theta (4–10 Hz) band pass. (B) Firing probability as a function of theta phase (gray bars: average; colored traces represent individual cells; color codes for individual cells within the PVBC and OLM cell classes are consistent throughout the figures; n = 7). The top trace is an idealized LFP theta (peaks, 180°/540°; trough, 360°/720°); note that the preferential firing (peak of gray bars) occurs before the trough. (C) Activity of the cell in A during immobility (same filters as in A); note the irregular firing and LFP. (D) Firing frequency of PVBCs during resting and running periods. All cells increased their discharge rate during running. (E) Camera lucida reconstruction of the biocytin-filled PVBC whose activity is shown in A and C. Axons (partially reconstructed from three 60-μm sections) are blue, and dendrites (fully reconstructed) are red. l-m, lacunosum moleculare; or, oriens; pyr, pyramidal; rad, radiatum; str. stratum. (Inset) Photomicrograph of the soma and the proximal dendrites of the illustrated cell after conversion of the fluorescent biocytin label to a more permanent signal with DAB. (F) (Left) parvalbumin-positive axon terminal of the PVBC shown in E. BIO, biocytin. (Right) Representative electron microscopic image shows an axon terminal of the same cell; the synaptic contact (arrow) was on a soma (S) in stratum pyramidale. (Scale bars: A and C: vertical: 0.5 mV; horizontal: 200 ms; E: 100 μm; Inset in E: 10 μm; F: light microscopy: 5 μm, electron microscopy: 0.5 μm.)
Fig. 2.
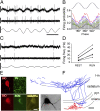
Entrainment of OLM cells during theta rhythm. (A) Single-unit activity during running-associated theta oscillations. Filter settings for the illustrated traces are as in Fig. 1. (B) Firing probability as a function of theta phase (gray bars: average; colored traces represent individual cells). (C) Firing of the OLM cell in A during immobility. (D) Firing frequency of OLM cells during resting and running periods. (E) Immunopositivity of the cell shown in A and C for somatostatin and mGluR1a but not for parvalbumin. (F) Reconstruction of the same cell (axons: blue, partly reconstructed from five 60-μm sections; dendrites: red, complete reconstruction). (Inset) Photomicrograph of the soma and the proximal dendrites of the illustrated cell after conversion of the fluorescent biocytin label to a more permanent signal with DAB (image taken at a different focal plane than in E). (Scale bars in A and C: vertical, 0.5 mV, horizontal, 200 ms; scale bars in E: 10 μm; scale bars in F: 100 μm.)
Fig. 3.
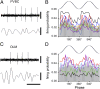
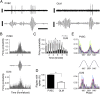
Firing of identified PVBCs and OLM cells during gamma oscillations. (A and C) (Upper) Extracellular unit recordings during gamma oscillations and (Lower) LFP (25–90 Hz band pass-filtered) recordings. LFP was recorded extracellularly with a second electrode in the stratum pyramidale from an identified PVBC (A) and an OLM cell (C). (B and D) Firing probability as a function of gamma phase (gray bars: average; different colors indicate individual cells) from PVBCs (B) and OLM cells (D). Note that all PVBCs (n = 7) were phase locked close to the midpoint of the ascending phase of the gamma oscillation; two of the five OLM cells did not show phase locking, and the three OLM cells that were phase locked fired later than the PVBCs during individual gamma waves. (Scale bars in A and C: unit recordings, 0.5 mV; LFP, 0.2 mV; time scale, 50 ms.)
Fig. 6.
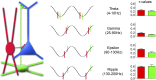
Summary illustration of the oscillation frequency-independent sequential GABAergic inputs to the perisomatic region and distal apical dendrites of CA1 pyramidal cells delivered by PVBCs and OLM cells. (Left) A pyramidal cell (blue); PVBC (red); and OLM cell (green). (Center) Summary of the mean preferential phase locking of PVBCs (red) and OLM cells (green) to theta, gamma, epsilon, and ripple oscillations. (Right) Magnitude of phase locking of PVBCs and OLM cells. Note that for both cell types the strongest phase locking occurred during ripples, and the weakest occurred during gamma oscillations.
Fig. 4.
Phase locking of PVBCs and OLM cells to epsilon oscillations. (A and C) (Upper) Extracellular unit recordings during epsilon oscillations. (Lower) LFP trace (90–130 Hz band-pass filtered) recorded with a separate electrode in the stratum pyramidale from a PVBC (A) and an OLM cell (C). (B and D) Firing probability as a function of epsilon phase (gray bars: average; different colors indicate individual cells) from PVBCs (B) and OLM cells (D). Note that the PVBCs preferentially fire during the early ascending phase, whereas OLM cells fire during the late ascending phase of the epsilon waves. (Scale bars in A and C: unit recordings, 1 mV; LFP, 0.2 mV; time scale, 10 ms.)
Fig. 5.
Interneuronal spiking during ripple oscillations in the awake mouse. (A) Unit recordings (Upper) during ripple oscillations (Lower) from a PVBC (Left) and an OLM cell (Right). (B) Firing probability as a function of normalized time (start of ripple: −1; end of ripple: +1) for PVBCs (Upper) and OLM cells (Lower). (C) Cell type-specific temporal evolution of firing probability during ripples. *P < 0.05; Bonferroni’s multiple comparisons test. (D) Percent of ripples during which PVBCs or OLM cells fired at least one spike. Note that PVCBs fired during most ripples, whereas OLM cells fired during about half of the ripples. *P < 0.05; Bonferroni’s multiple comparisons test. (E) Firing probability as a function of the phase of the ripple wave (gray bars: average; different colors indicate individual cells) from PVBCs (Upper) and OLM cells (Lower). (Scale bars: unit recordings, 0.5 mV; LFPs, 0.2 mV; time scale, 100 ms.)
Fig. P1.
Simultaneous electrophysiological recordings of action potential discharges in post hoc-identified GABAergic interneurons and LFPs during state-dependent hippocampal network oscillations in awake animals revealed that perisomatically projecting inhibitory interneurons (red) fired before distal dendritically projecting cells (green), regardless of network oscillation frequency. These results demonstrate a temporal ordering of GABAergic cell activity that can modulate pyramidal cell discharges in the hippocampus during different behavioral states.
Similar articles
- Excitatory Inputs Determine Phase-Locking Strength and Spike-Timing of CA1 Stratum Oriens/Alveus Parvalbumin and Somatostatin Interneurons during Intrinsically Generated Hippocampal Theta Rhythm.
Huh CY, Amilhon B, Ferguson KA, Manseau F, Torres-Platas SG, Peach JP, Scodras S, Mechawar N, Skinner FK, Williams S. Huh CY, et al. J Neurosci. 2016 Jun 22;36(25):6605-22. doi: 10.1523/JNEUROSCI.3951-13.2016. J Neurosci. 2016. PMID: 27335395 Free PMC article. - Hippocampal Respiration-Driven Rhythm Distinct from Theta Oscillations in Awake Mice.
Nguyen Chi V, Müller C, Wolfenstetter T, Yanovsky Y, Draguhn A, Tort AB, Brankačk J. Nguyen Chi V, et al. J Neurosci. 2016 Jan 6;36(1):162-77. doi: 10.1523/JNEUROSCI.2848-15.2016. J Neurosci. 2016. PMID: 26740658 Free PMC article. - Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations.
Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Klausberger T, et al. J Neurosci. 2005 Oct 19;25(42):9782-93. doi: 10.1523/JNEUROSCI.3269-05.2005. J Neurosci. 2005. PMID: 16237182 Free PMC article. - GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations.
Cutsuridis V, Hasselmo M. Cutsuridis V, et al. Hippocampus. 2012 Jul;22(7):1597-621. doi: 10.1002/hipo.21002. Epub 2012 Jan 18. Hippocampus. 2012. PMID: 22252986 - Impaired mitochondrial function abolishes gamma oscillations in the hippocampus through an effect on fast-spiking interneurons.
Whittaker RG, Turnbull DM, Whittington MA, Cunningham MO. Whittaker RG, et al. Brain. 2011 Jul;134(Pt 7):e180; author reply e181. doi: 10.1093/brain/awr018. Epub 2011 Mar 4. Brain. 2011. PMID: 21378098 Free PMC article. Review. No abstract available.
Cited by
- High-frequency oscillations and sequence generation in two-population models of hippocampal region CA1.
Braun W, Memmesheimer RM. Braun W, et al. PLoS Comput Biol. 2022 Feb 17;18(2):e1009891. doi: 10.1371/journal.pcbi.1009891. eCollection 2022 Feb. PLoS Comput Biol. 2022. PMID: 35176028 Free PMC article. - [Neural mechanism for modulation of auditory response of the striatum by locomotion].
Huang W, Liang F. Huang W, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2022 May 20;42(5):766-771. doi: 10.12122/j.issn.1673-4254.2022.05.20. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 35673923 Free PMC article. Chinese. - Dynamic circuit motifs underlying rhythmic gain control, gating and integration.
Womelsdorf T, Valiante TA, Sahin NT, Miller KJ, Tiesinga P. Womelsdorf T, et al. Nat Neurosci. 2014 Aug;17(8):1031-9. doi: 10.1038/nn.3764. Epub 2014 Jul 28. Nat Neurosci. 2014. PMID: 25065440 Review. - Hippocampal Ripple Oscillations and Inhibition-First Network Models: Frequency Dynamics and Response to GABA Modulators.
Donoso JR, Schmitz D, Maier N, Kempter R. Donoso JR, et al. J Neurosci. 2018 Mar 21;38(12):3124-3146. doi: 10.1523/JNEUROSCI.0188-17.2018. Epub 2018 Feb 16. J Neurosci. 2018. PMID: 29453207 Free PMC article. - Excitation and inhibition compete to control spiking during hippocampal ripples: intracellular study in behaving mice.
English DF, Peyrache A, Stark E, Roux L, Vallentin D, Long MA, Buzsáki G. English DF, et al. J Neurosci. 2014 Dec 3;34(49):16509-17. doi: 10.1523/JNEUROSCI.2600-14.2014. J Neurosci. 2014. PMID: 25471587 Free PMC article.
References
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. - PubMed
- Buzsáki G, Leung LW, Vanderwolf CH. Cellular bases of hippocampal EEG in the behaving rat. Brain Res. 1983;287:139–171. - PubMed
- Cohen NJ, Eichenbaum H. The theory that wouldn’t die: A critical look at the spatial mapping theory of hippocampal function. Hippocampus. 1991;1:265–268. - PubMed
- McNaughton N, Ruan M, Woodnorth MA. Restoring theta-like rhythmicity in rats restores initial learning in the Morris water maze. Hippocampus. 2006;16:1102–1110. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS035915/NS/NINDS NIH HHS/United States
- I01 BX001524/BX/BLRD VA/United States
- R37 NS035915/NS/NINDS NIH HHS/United States
- NS35915/NS/NINDS NIH HHS/United States
- K08 NS056210/NS/NINDS NIH HHS/United States
- K08 NS0526210/NS/NINDS NIH HHS/United States
- R29 NS035915/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous