Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis - PubMed (original) (raw)
Review
. 2012 Nov 10;380(9854):1662-73.
doi: 10.1016/S0140-6736(12)61350-6. Epub 2012 Sep 24.
Kunihiro Matsushita, Mark Woodward, Henk J G Bilo, John Chalmers, Hiddo J Lambers Heerspink, Brian J Lee, Robert M Perkins, Peter Rossing, Toshimi Sairenchi, Marcello Tonelli, Joseph A Vassalotti, Kazumasa Yamagishi, Josef Coresh, Paul E de Jong, Chi-Pang Wen, Robert G Nelson; Chronic Kidney Disease Prognosis Consortium
Collaborators, Affiliations
- PMID: 23013602
- PMCID: PMC3771350
- DOI: 10.1016/S0140-6736(12)61350-6
Review
Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis
Caroline S Fox et al. Lancet. 2012.
Erratum in
- Lancet. 2013 Feb 2;381(9864):374
Abstract
Background: Chronic kidney disease is characterised by low estimated glomerular filtration rate (eGFR) and high albuminuria, and is associated with adverse outcomes. Whether these risks are modified by diabetes is unknown.
Methods: We did a meta-analysis of studies selected according to Chronic Kidney Disease Prognosis Consortium criteria. Data transfer and analyses were done between March, 2011, and June, 2012. We used Cox proportional hazards models to estimate the hazard ratios (HR) of mortality and end-stage renal disease (ESRD) associated with eGFR and albuminuria in individuals with and without diabetes.
Findings: We analysed data for 1,024,977 participants (128,505 with diabetes) from 30 general population and high-risk cardiovascular cohorts and 13 chronic kidney disease cohorts. In the combined general population and high-risk cohorts with data for all-cause mortality, 75,306 deaths occurred during a mean follow-up of 8·5 years (SD 5·0). In the 23 studies with data for cardiovascular mortality, 21,237 deaths occurred from cardiovascular disease during a mean follow-up of 9·2 years (SD 4·9). In the general and high-risk cohorts, mortality risks were 1·2-1·9 times higher for participants with diabetes than for those without diabetes across the ranges of eGFR and albumin-to-creatinine ratio (ACR). With fixed eGFR and ACR reference points in the diabetes and no diabetes groups, HR of mortality outcomes according to lower eGFR and higher ACR were much the same in participants with and without diabetes (eg, for all-cause mortality at eGFR 45 mL/min per 1·73 m(2) [vs 95 mL/min per 1·73 m(2)], HR 1·35; 95% CI 1·18-1·55; vs 1·33; 1·19-1·48 and at ACR 30 mg/g [vs 5 mg/g], 1·50; 1·35-1·65 vs 1·52; 1·38-1·67). The overall interactions were not significant. We identified much the same findings for ESRD in the chronic kidney disease cohorts.
Interpretation: Despite higher risks for mortality and ESRD in diabetes, the relative risks of these outcomes by eGFR and ACR are much the same irrespective of the presence or absence of diabetes, emphasising the importance of kidney disease as a predictor of clinical outcomes.
Funding: US National Kidney Foundation.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
KM’s institution has received grants from the US National Kidney Foundation, Amgen, and US National Institutes of Health. MW’s institution has received a grant from Servier; he has received consultancy fees from Roche and speaker’s fees from Sanofi and Servier. JCh’s institution has received grants from Servier; and he has received speaker’s fees from them. HJLH has served as a consultant for Abbott, Reata, Johnson & Johnson, and Vitae; and he has received speaker’s fees from Abbott. BJL has received travel expenses from Kidney Disease: Improving Global Outcomes. RMP has received grants from the US National Kidney Foundation. PR’s institution has received consultancy fees from Abbott, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, and Novo Nordisk, and has received grants from Abbott, Novartis, Pfizer, and speaker’s fees from Abbott, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, and Novo Nordisk; he holds stock in Novo Nordisk. JAV has received consultancy fees from CTI Clinical Trial Services and Litholink Chronic Kidney Disease Advisory Board; his institution has received grants from the US National Institutes of Health; and he has received speaker’s fees from Gore Creative Technologies and Elsevier. JCo’s institution has received grants from the US National Kidney Foundation, US National Institutes of Health, and Amgen, and has received consultancy fees from Amgen and Merck. PEdJ’s institution has received a grant from the Dutch Kidney Foundation. CSF, HJGB, TS, MT, KY, C-PW and RGN declare that they have no conflicts of interest.
Figures
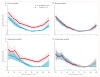
Figure 1. Hazard ratios for all-cause and cardiovascular mortality in the combined general and high-risk populations according to eGFR in individuals with and without diabetes
(A, B) All-cause mortality. (C, D) Cardiovascular mortality. Panels A and C use one reference point (diamond, eGFR of 95 mL/min per 1·73 m2 in the no diabetes group) for both individuals with and without diabetes to show the main effect of diabetes on risk. Panels B and D use separate references (diamonds) in the diabetes and no diabetes groups to assess interaction with diabetes specifically. Hazard ratios were adjusted for age, sex, race, smoking, history of cardiovascular disease, serum total cholesterol concentration, body-mass index, and albuminuria (log albumin-to-creatinine ratio, log protein-to-creatinine, or categorical dipstick proteinuria [negative, trace, 1+, ≥2+]). Blue and red circles denote p<0·05 as compared with the reference (diamond). Significant interaction between diabetes and eGFR is shown by x signs. eGFR=estimated glomerular filtration rate.
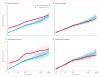
Figure 2. Hazard ratios for all-cause and cardiovascular mortality in the combined general and high-risk populations according to ACR in participants with and without diabetes
(A, B) All-cause mortality. (C, D) Cardiovascular mortality. Panels A and C use one reference point (diamond, ACR of 5 mg/g in the no diabetes group), for both individuals with and without hypertension to show the main effect of diabetes on risk. Panels B and D use separate references (diamonds) in the diabetes and no diabetes groups to assess interaction with diabetes specifically. Hazard ratios were adjusted for age, sex, race, smoking, history of cardiovascular disease, serum total cholesterol concentration, body-mass index, and estimated glomerular filtration rate. Blue and red circles denote p<0·05 as compared with the reference (diamond). Significant interaction between diabetes and ACR is shown by x signs. ACR=albumin-to-creatinine ratio.
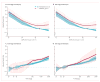
Figure 3. Hazard ratios for end-stage renal disease in the chronic kidney disease populations according to eGFR and ACR in participants with and without diabetes
(A, B) eGFR. (C, D) ACR. Panels A and C use eGFR of 50 mL/min per 1·73 m2 (A) and ACR of 20 mg/g (C) in individuals without diabetes as the reference point (diamond) for both individuals with and without diabetes. Panels B and D use eGFR of 50 mL/min per 1·73 m2 (B) and ACR of 20 mg/g (D) as the reference points (diamond) in diabetic and non-diabetic groups. Blue and red circles denote p<0·05 as compared with the reference (diamond). Hazard ratios were adjusted for age, sex, race, smoking, history of cardiovascular disease, serum total cholesterol concentration, body-mass index, and albuminuria (log albumin-to-creatinine ratio, log protein-to-creatinine, or categorical dipstick proteinuria [negative/ trace, 1+, 2+, ≥3+]) or eGFR. eGFR=estimated glomerular filtration rate. ACR=albumin-to-creatinine ratio.
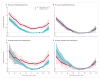
Figure 4. Hazard ratios for all-cause mortality according to eGFR in participants with and without diabetes in individuals with and without hypertension from the general population and high-risk cohorts
(A, B) Individuals with hypertension. (C, D) Individuals without hypertension. Blue and red circles denote p<0·05 as compared with the reference (diamond). Significant interaction between diabetes and eGFR is shown by x signs. Hazard ratios were adjusted for age, sex, race, smoking, history of cardiovascular disease, serum total cholesterol concentration, body-mass index, and albuminuria (log albumin-to-creatinine ratio, log protein-to-creatinine, or categorical dipstick proteinuria [negative, trace, 1+, ≥2+])
Comment in
- Association of kidney disease measures with poor outcomes.
Stevens PE, Farmer CK. Stevens PE, et al. Lancet. 2012 Nov 10;380(9854):1628-30. doi: 10.1016/S0140-6736(12)61300-2. Epub 2012 Sep 24. Lancet. 2012. PMID: 23013601 No abstract available. - Chronic kidney disease: Association of chronic kidney disease with adverse outcomes in the absence of hypertension and diabetes.
Allison SJ. Allison SJ. Nat Rev Nephrol. 2012 Nov;8(11):611. doi: 10.1038/nrneph.2012.199. Epub 2012 Oct 9. Nat Rev Nephrol. 2012. PMID: 23045229 No abstract available. - Association of chronic kidney disease with adverse outcomes.
Du X, Zhao Y, Huang W, Liu L, Cao C. Du X, et al. Lancet. 2013 Feb 16;381(9866):531-2. doi: 10.1016/S0140-6736(13)60272-X. Lancet. 2013. PMID: 23415295 No abstract available. - Association of chronic kidney disease with adverse outcomes.
Izumi Y, Ogawa M, Itoh H, Shimada H, Nonoguchi H. Izumi Y, et al. Lancet. 2013 Feb 16;381(9866):531. doi: 10.1016/S0140-6736(13)60271-8. Lancet. 2013. PMID: 23415296 No abstract available. - Association of chronic kidney disease with adverse outcomes - Authors' reply.
Mahmoodi BK, Fox CS, Astor BC, Nelson RG, Matsushita K, Coresh J; Chronic Kidney Disease Prognosis Consortium. Mahmoodi BK, et al. Lancet. 2013 Feb 16;381(9866):532-3. doi: 10.1016/S0140-6736(13)60273-1. Lancet. 2013. PMID: 23415297 Free PMC article. No abstract available.
Similar articles
- Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis.
Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, Rossing P, Sarnak MJ, Stengel B, Yamagishi K, Yamashita K, Zhang L, Coresh J, de Jong PE, Astor BC; Chronic Kidney Disease Prognosis Consortium. Mahmoodi BK, et al. Lancet. 2012 Nov 10;380(9854):1649-61. doi: 10.1016/S0140-6736(12)61272-0. Epub 2012 Sep 24. Lancet. 2012. PMID: 23013600 Free PMC article. Review. - Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death.
Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL. Berhane AM, et al. Clin J Am Soc Nephrol. 2011 Oct;6(10):2444-51. doi: 10.2215/CJN.00580111. Epub 2011 Aug 18. Clin J Am Soc Nephrol. 2011. PMID: 21852671 Free PMC article. - Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all-cause mortality in Japanese patients with type 2 diabetes.
Wada T, Haneda M, Furuichi K, Babazono T, Yokoyama H, Iseki K, Araki S, Ninomiya T, Hara S, Suzuki Y, Iwano M, Kusano E, Moriya T, Satoh H, Nakamura H, Shimizu M, Toyama T, Hara A, Makino H; Research Group of Diabetic Nephropathy, Ministry of Health, Labour, and Welfare of Japan. Wada T, et al. Clin Exp Nephrol. 2014 Aug;18(4):613-20. doi: 10.1007/s10157-013-0879-4. Epub 2013 Oct 17. Clin Exp Nephrol. 2014. PMID: 24132561 - Age and association of kidney measures with mortality and end-stage renal disease.
Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J; Chronic Kidney Disease Prognosis Consortium. Hallan SI, et al. JAMA. 2012 Dec 12;308(22):2349-60. doi: 10.1001/jama.2012.16817. JAMA. 2012. PMID: 23111824 Free PMC article. - Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta-analysis.
Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, Oien CM, Levey AS, Levin A, Woodward M, Hemmelgarn BR; Chronic Kidney Disease Prognosis Consortium. Nitsch D, et al. BMJ. 2013 Jan 29;346:f324. doi: 10.1136/bmj.f324. BMJ. 2013. PMID: 23360717 Free PMC article. Review.
Cited by
- Cardiovascular and renal outcomes by baseline albuminuria status and renal function: Results from the LEADER randomized trial.
Mosenzon O, Bain SC, Heerspink HJL, Idorn T, Mann JFE, Persson F, Pratley RE, Rasmussen S, Rossing P, von Scholten BJ, Raz I; LEADER Trial Investigators. Mosenzon O, et al. Diabetes Obes Metab. 2020 Nov;22(11):2077-2088. doi: 10.1111/dom.14126. Epub 2020 Aug 7. Diabetes Obes Metab. 2020. PMID: 32618386 Free PMC article. Clinical Trial. - Polyphenols mediated attenuation of diabetes associated cardiovascular complications: A comprehensive review.
Kour N, Bhagat G, Singh S, Bhatti SS, Arora S, Singh B, Bhatia A. Kour N, et al. J Diabetes Metab Disord. 2023 Oct 18;23(1):73-99. doi: 10.1007/s40200-023-01326-x. eCollection 2024 Jun. J Diabetes Metab Disord. 2023. PMID: 38932901 Free PMC article. Review. - Diabetic nephropathy in Africa: A systematic review.
Noubiap JJ, Naidoo J, Kengne AP. Noubiap JJ, et al. World J Diabetes. 2015 Jun 10;6(5):759-73. doi: 10.4239/wjd.v6.i5.759. World J Diabetes. 2015. PMID: 26069725 Free PMC article. - Novel Insights into Diabetic Kidney Disease.
Młynarska E, Buławska D, Czarnik W, Hajdys J, Majchrowicz G, Prusinowski F, Stabrawa M, Rysz J, Franczyk B. Młynarska E, et al. Int J Mol Sci. 2024 Sep 23;25(18):10222. doi: 10.3390/ijms251810222. Int J Mol Sci. 2024. PMID: 39337706 Free PMC article. Review. - Chronic kidney disease in adults aged 18 years and older in Chile: findings from the cross-sectional Chilean National Health Surveys 2009-2010 and 2016-2017.
Walbaum M, Scholes S, Pizzo E, Paccot M, Mindell JS. Walbaum M, et al. BMJ Open. 2020 Sep 3;10(9):e037720. doi: 10.1136/bmjopen-2020-037720. BMJ Open. 2020. PMID: 32883732 Free PMC article.
References
- Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–99. - PubMed
- Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–40. - PubMed
- Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. - PubMed
- Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–84. - PubMed
- Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol. 2003;14(suppl 2):S131–38. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 DK035073/DK/NIDDK NIH HHS/United States
- HHSN268201100010C/HL/NHLBI NIH HHS/United States
- HHSN268201100011C/HL/NHLBI NIH HHS/United States
- R01 AG007181/AG/NIA NIH HHS/United States
- R01 DK073217/DK/NIDDK NIH HHS/United States
- HHSN268201100006C/HL/NHLBI NIH HHS/United States
- U01 NS041588/NS/NINDS NIH HHS/United States
- N01 HC095169/HC/NHLBI NIH HHS/United States
- R01 HL080295/HL/NHLBI NIH HHS/United States
- R01 AG020098/AG/NIA NIH HHS/United States
- HHSN268201100007I/HL/NHLBI NIH HHS/United States
- N01 HC035129/HC/NHLBI NIH HHS/United States
- CZH/4/656/CSO_/Chief Scientist Office/United Kingdom
- N01 HC095159/HC/NHLBI NIH HHS/United States
- HHSN268201100012C/HL/NHLBI NIH HHS/United States
- K23 DK067303/DK/NIDDK NIH HHS/United States
- N01 HC085086/HC/NHLBI NIH HHS/United States
- R01 HL043232/HL/NHLBI NIH HHS/United States
- HHSN268201100009I/HL/NHLBI NIH HHS/United States
- R01 AG015928/AG/NIA NIH HHS/United States
- HHSN268201100008C/HL/NHLBI NIH HHS/United States
- HHSN268201100005G/HL/NHLBI NIH HHS/United States
- N01 HC075150/HC/NHLBI NIH HHS/United States
- HHSN268201100008I/HL/NHLBI NIH HHS/United States
- K23 DK002904/DK/NIDDK NIH HHS/United States
- U10 EY006594/EY/NEI NIH HHS/United States
- HHSN268201100007C/HL/NHLBI NIH HHS/United States
- N01 HC015103/HC/NHLBI NIH HHS/United States
- N01 HC025195/HC/NHLBI NIH HHS/United States
- HHSN268201100011I/HL/NHLBI NIH HHS/United States
- HHSN268201200036C/HL/NHLBI NIH HHS/United States
- R01 DK031801/DK/NIDDK NIH HHS/United States
- N01 HC055222/HL/NHLBI NIH HHS/United States
- HHSN268201100005I/HL/NHLBI NIH HHS/United States
- HHSN268201100009C/HL/NHLBI NIH HHS/United States
- HHSN268201100005C/HL/NHLBI NIH HHS/United States
- N01 HC085079/HC/NHLBI NIH HHS/United States
- N01 HC075150/HL/NHLBI NIH HHS/United States
- R01 HL068140/HL/NHLBI NIH HHS/United States
- R01 AG023629/AG/NIA NIH HHS/United States
- R01 AG028507/AG/NIA NIH HHS/United States
- R01 AG027058/AG/NIA NIH HHS/United States
- N01 HC045133/HC/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous