TRPA1: A gatekeeper for inflammation - PubMed (original) (raw)
Review
TRPA1: A gatekeeper for inflammation
Diana M Bautista et al. Annu Rev Physiol. 2013.
Abstract
Tissue damage evokes an inflammatory response that promotes the removal of harmful stimuli, tissue repair, and protective behaviors to prevent further damage and encourage healing. However, inflammation may outlive its usefulness and become chronic. Chronic inflammation can lead to a host of diseases, including asthma, itch, rheumatoid arthritis, and colitis. Primary afferent sensory neurons that innervate target organs release inflammatory neuropeptides in the local area of tissue damage to promote vascular leakage, the recruitment of immune cells, and hypersensitivity to mechanical and thermal stimuli. TRPA1 channels are required for neuronal excitation, the release of inflammatory neuropeptides, and subsequent pain hypersensitivity. TRPA1 is also activated by the release of inflammatory agents from nonneuronal cells in the area of tissue injury or disease. This dual function of TRPA1 as a detector and instigator of inflammatory agents makes TRPA1 a gatekeeper of chronic inflammatory disorders of the skin, airways, and gastrointestinal tract.
Figures
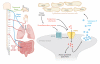
Figure 1
Mechanisms of neurogenic inflammation. (Left) TRPA1 is expressed by sensory afferents that have cell bodies in the vagus nerve (blue), trigeminal ganglia ( green), and dorsal root ganglia (red ). These afferents innervate peripheral targets, including the skin, the airways, and the GI tract. (Right) TRPA1 activation is required for the release of neuropeptides such as substance P, calcitonin gene-related peptide (CGRP), and neurokinin A (NKA), which promote and modulate inflammatory responses.
Figure 2
Diverse mechanisms of TRPA1 activation. Endogenous and exogenous agonists covalently modify cysteines ( yellow; C) in the TRPA1 N terminus to promote channel activity. In addition, signaling molecules downstream of G protein–coupled receptors (GPCRs) regulate TRPA1 channel activity. Other putative binding/modulatory sites that regulate channel activity include ankyrin domains ( gray), calcium-binding domains (red; D), the familial episodic pain syndrome (FEPS) mutation ( green; N), and zinc-binding sites (orange; C, H). Additional TRPA1 domains for activation by phospholipase C (PLC) and Gβγ are not known.
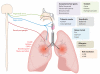
Figure 3
TRPA1 in airway inflammation. The respiratory system is densely innervated by TRPA1-expressing primary afferent fibers from the trigeminal nerve, vagus nerve, and dorsal root ganglia. TRPA1 is activated by numerous exogenous irritants and endogenous mediators of airway inflammation. TRPA1 activation in the airways has been linked to cough, asthma, chronic obstructive pulmonary disease (COPD), bronchospasm, and mucus secretion.
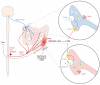
Figure 4
TRPA1-expressing sensory nerves innervate the colon and mediate neuropeptide release in response to TRPA1 activators such as 4-hydroxynonenal (4-HNE). Experimental colitis models induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) or dextran sodium sulfate (DSS) lead to hypersensitivity to colorectal distension (CRD) and pain.
Similar articles
- Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation.
Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, Viscomi AR, Pisano AR, Stokesberry S, Brunmark C, Svitacheva N, McGarvey L, Patacchini R, Damholt AB, Geppetti P, Materazzi S. Nassini R, et al. PLoS One. 2012;7(8):e42454. doi: 10.1371/journal.pone.0042454. Epub 2012 Aug 14. PLoS One. 2012. PMID: 22905134 Free PMC article. - TRPA1 channels: molecular sentinels of cellular stress and tissue damage.
Viana F. Viana F. J Physiol. 2016 Aug 1;594(15):4151-69. doi: 10.1113/JP270935. J Physiol. 2016. PMID: 27079970 Free PMC article. Review. - Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control.
Bessac BF, Jordt SE. Bessac BF, et al. Physiology (Bethesda). 2008 Dec;23:360-70. doi: 10.1152/physiol.00026.2008. Physiology (Bethesda). 2008. PMID: 19074743 Free PMC article. Review. - The TRPA1 channel in inflammatory and neuropathic pain and migraine.
Nassini R, Materazzi S, Benemei S, Geppetti P. Nassini R, et al. Rev Physiol Biochem Pharmacol. 2014;167:1-43. doi: 10.1007/112_2014_18. Rev Physiol Biochem Pharmacol. 2014. PMID: 24668446 Review. - Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles.
Kun J, Szitter I, Kemény A, Perkecz A, Kereskai L, Pohóczky K, Vincze A, Gódi S, Szabó I, Szolcsányi J, Pintér E, Helyes Z. Kun J, et al. PLoS One. 2014 Sep 29;9(9):e108164. doi: 10.1371/journal.pone.0108164. eCollection 2014. PLoS One. 2014. PMID: 25265225 Free PMC article.
Cited by
- Inhibition of inflammatory pain and cough by a novel charged sodium channel blocker.
Tochitsky I, Jo S, Andrews N, Kotoda M, Doyle B, Shim J, Talbot S, Roberson D, Lee J, Haste L, Jordan SM, Levy BD, Bean BP, Woolf CJ. Tochitsky I, et al. Br J Pharmacol. 2021 Oct;178(19):3905-3923. doi: 10.1111/bph.15531. Epub 2021 Jun 21. Br J Pharmacol. 2021. PMID: 33988876 Free PMC article. - Finding disease modules for cancer and COVID-19 in gene co-expression networks with the Core&Peel method.
Lucchetta M, Pellegrini M. Lucchetta M, et al. Sci Rep. 2020 Oct 19;10(1):17628. doi: 10.1038/s41598-020-74705-6. Sci Rep. 2020. PMID: 33077837 Free PMC article. - Transient receptor potential channels in pulmonary chemical injuries and as countermeasure targets.
Achanta S, Jordt SE. Achanta S, et al. Ann N Y Acad Sci. 2020 Nov;1480(1):73-103. doi: 10.1111/nyas.14472. Epub 2020 Sep 6. Ann N Y Acad Sci. 2020. PMID: 32892378 Free PMC article. - Platycodonis Radix Alleviates LPS-Induced Lung Inflammation through Modulation of TRPA1 Channels.
Yang T, Zhao S, Yuan Y, Zhao X, Bu F, Zhang Z, Li Q, Li Y, Wei Z, Sun X, Zhang Y, Xie J. Yang T, et al. Molecules. 2023 Jul 5;28(13):5213. doi: 10.3390/molecules28135213. Molecules. 2023. PMID: 37446875 Free PMC article. - Peripheral Neuroinflammation and Pain: How Acute Pain Becomes Chronic.
Schumacher MA. Schumacher MA. Curr Neuropharmacol. 2024;22(1):6-14. doi: 10.2174/1570159X21666230808111908. Curr Neuropharmacol. 2024. PMID: 37559537 Free PMC article. Review.
References
- Basbaum AI, Woolf CJ. Pain. Curr. Biol. 1999;9:R429–31. - PubMed
- Meyer RA, Ringkamp M, Campbell JN, Raja SN. Peripheral mechanisms of cutaneous nociception. In: MacMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Elsevier; Philadelphia: 2006. pp. 3–34.
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–29. - PubMed
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials