An RMND1 Mutation causes encephalopathy associated with multiple oxidative phosphorylation complex deficiencies and a mitochondrial translation defect - PubMed (original) (raw)
An RMND1 Mutation causes encephalopathy associated with multiple oxidative phosphorylation complex deficiencies and a mitochondrial translation defect
Alexandre Janer et al. Am J Hum Genet. 2012.
Abstract
Mutations in the genes composing the mitochondrial translation apparatus are an important cause of a heterogeneous group of oxidative phosphorylation (OXPHOS) disorders. We studied the index case in a consanguineous family in which two children presented with severe encephalopathy, lactic acidosis, and intractable seizures leading to an early fatal outcome. Blue native polyacrylamide gel electrophoretic (BN-PAGE) analysis showed assembly defects in all of the OXPHOS complexes with mtDNA-encoded structural subunits, and these defects were associated with a severe deficiency in mitochondrial translation. Immunoblot analysis showed reductions in the steady-state levels of several structural subunits of the mitochondrial ribosome. Whole-exome sequencing identified a homozygous missense mutation (c.1250G>A) in an uncharacterized gene, RMND1 (required for meiotic nuclear division 1). RMND1 localizes to mitochondria and behaves as an integral membrane protein. Retroviral expression of the wild-type RMND1 cDNA rescued the biochemical phenotype in subject cells, and siRNA-mediated knockdown of the protein recapitulated the defect. BN-PAGE, gel filtration, and mass spectrometry analyses showed that RMND1 forms a high-molecular-weight and most likely homopolymeric complex (∼240 kDa) that does not assemble in subject fibroblasts but that is rescued by expression of RMND1 cDNA. The p.Arg417Gln substitution, predicted to be in a coiled-coil domain, which is juxtaposed to a transmembrane domain at the extreme C terminus of the protein, does not alter the steady-state level of RMND1 but might prevent protein-protein interactions in this complex. Our results demonstrate that the RMND1 complex is necessary for mitochondrial translation, possibly by coordinating the assembly or maintenance of the mitochondrial ribosome.
Copyright © 2012 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
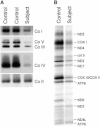
Figure 1
Characterization of Biochemical and Molecular Defects in Subject Fibroblasts Control and subject fibroblasts were analyzed by BN-PAGE (A) and by pulse labeling of the mtDNA-encoded polypeptides (B). (A) Each of the five OXPHOS complexes (I–V) was visualized with a subunit-specific antibody that recognizes the native complex as follows: CoI (NDUFA9), CoII (SDHA), CoIII (UQCRC1), CoIV (COX4), CoV (ATP5A1). Complex II is the loading control. (B) The seven subunits of complex I (ND), one subunit of complex III (cyt b), three subunits of complex IV (COX), and two subunits of complex V (ATP) are indicated to the right of the figure.
Figure 2
Steady-State Levels of Mitochondrial DNA, mRNAs, rRNAs, and Mitochondrial-Translation Proteins (A) Southern blot analysis of genomic DNA extracted from control and subject fibroblasts. Hybridization was performed with probes directed against a 16 kb fragment of the mitochondrial genome, and the nuclear 18S rRNA gene was used as a loading control. (B) Northern blot analysis carried out with total RNA extracted from control and subject fibroblasts. Hybridization was performed with probes specific to mitochondrial mRNAs encoding the three COX subunits, one of the complex I subunits (ND1), and the 12S and 16S mitochondrial ribosomal RNAs. Beta-actin was used as the loading control. (C) Immunoblot analysis of control and subject fibroblasts with antibodies against the mitochondrial-translation elongation factors (EFG1and EFTs) and the mitochondrial ribosomal proteins MRPL32 (a kind gift of T. Langer, Cologne), MRPL13, MRPL15, and MRPS2 (kind gifts of L. Spremulli, UNC Chapel Hill). The 70 kDa subunit of complex II (SDHA) was used as a loading control.
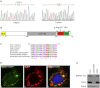
Figure 3
Mutational Analysis of RMND1 and Mitochondrial Localization of the Protein (A) DNA sequence analysis of RMND1 cDNA indicates the position of the homozygous c.1250G>A mutation in the subject compared to the control. (B) A schematic representation of RMND1 (not to scale) shows the predicted domains and the position of the p.Arg417Gln substitution. The following abbreviations are used: MLS, mitochondrial localization signal; DUF155, domain of unknown function 155; CC, coiled-coil domain; and TM, transmembrane domain. (C) The alignment of the amino acid sequences of RMND1 homologs in different species shows that the mutated arginine (black rectangle) is conserved only in the vertebrates. (D) Control fibroblasts transiently expressing RMND1-EGFP (left panel, green) were incubated with an antibody against the mitochondrial protein SLIRP (middle panel, red). The far right panel showing the overlay is counterstained with DAPI for visualization of the nucleus. (E) Alkaline carbonate extraction of mitochondria from HEK cells stably expressing a C-terminal Myc-tagged RMND1. Immunoblot analysis with an Myc antibody shows that RMND1 is an integral membrane protein. SDHA (soluble, membrane-associated protein) and COX subunit 2 (integral inner membrane protein) were used as controls.
Figure 4
Rescue of the Biochemical Phenotype by RMND1 Expression and Recapitulation of the Defect by siRNA-Mediated Knockdown of the Protein (A) BN-PAGE analysis of controls and subject fibroblasts expressing RMND1 from a retroviral vector (pBABE). Each of the five OXPHOS complexes (I–V) was visualized with a subunit-specific antibody. (B) Immunoblot analysis of the same samples as in (A) for expression of RMND1, individual structural subunits of the five OXPHOS complexes, and two mitochondrial ribosomal subunits. The 70 kDa subunit of complex II (SDHA) was used as a loading control. (C) Analysis of mitochondrial translation products in control and subject fibroblasts transduced with retroviral vectors expressing RMND1-HA or RMND1-flag. (D) Stealth RNAi-mediated knockdown of RMND1. The upper panel shows the level of knockdown of RMND1 on an immunoblot with VDAC1 (porin) as a loading control. The bottom panel shows the BN-PAGE analysis in the control, RMND1 knockdown, and subject cells.
Figure 5
RMND1 Is Part of a High-Molecular-Weight Protein Complex that Does Not Assemble in the Subject (A) Size-exclusion-chromatography analysis was carried out with mitochondria from HEK cells expressing RMND1-Myc. Immunoblotting with antibodies against either the native protein or the Myc epitope demonstrated that both the endogenous and overexpressed proteins are part of a complex of about 240 kDa. The COX subunit 1 of COX (230 kDa) and LRPPRC (250 kDa) were used as molecular-weight references. (B) BN-PAGE analysis of control and subject fibroblasts expressing RMND1-Myc from a retroviral vector. An antibody directed against the native protein shows that RMND1 forms a 250 kDa complex that does not assemble in the subject. The complex is restored in subject cells expressing RMND1-Myc. SDHA was used as a loading control.
Similar articles
- Mitochondrial DNA transcription and translation: clinical syndromes.
Boczonadi V, Ricci G, Horvath R. Boczonadi V, et al. Essays Biochem. 2018 Jul 20;62(3):321-340. doi: 10.1042/EBC20170103. Print 2018 Jul 20. Essays Biochem. 2018. PMID: 29980628 Free PMC article. Review. - Infantile encephaloneuromyopathy and defective mitochondrial translation are due to a homozygous RMND1 mutation.
Garcia-Diaz B, Barros MH, Sanna-Cherchi S, Emmanuele V, Akman HO, Ferreiro-Barros CC, Horvath R, Tadesse S, El Gharaby N, DiMauro S, De Vivo DC, Shokr A, Hirano M, Quinzii CM. Garcia-Diaz B, et al. Am J Hum Genet. 2012 Oct 5;91(4):729-36. doi: 10.1016/j.ajhg.2012.08.019. Epub 2012 Sep 27. Am J Hum Genet. 2012. PMID: 23022099 Free PMC article. - RMND1 deficiency associated with neonatal lactic acidosis, infantile onset renal failure, deafness, and multiorgan involvement.
Janer A, van Karnebeek CD, Sasarman F, Antonicka H, Al Ghamdi M, Shyr C, Dunbar M, Stockler-Ispiroglu S, Ross CJ, Vallance H, Dionne J, Wasserman WW, Shoubridge EA. Janer A, et al. Eur J Hum Genet. 2015 Oct;23(10):1301-7. doi: 10.1038/ejhg.2014.293. Epub 2015 Jan 21. Eur J Hum Genet. 2015. PMID: 25604853 Free PMC article. - Hearing impairment and renal failure associated with RMND1 mutations.
Ravn K, Neland M, Wibrand F, Duno M, Ostergaard E. Ravn K, et al. Am J Med Genet A. 2016 Jan;170A(1):142-7. doi: 10.1002/ajmg.a.37399. Epub 2015 Sep 23. Am J Med Genet A. 2016. PMID: 26395190 - Mitochondrial transcription and translation: overview.
D'Souza AR, Minczuk M. D'Souza AR, et al. Essays Biochem. 2018 Jul 20;62(3):309-320. doi: 10.1042/EBC20170102. Print 2018 Jul 20. Essays Biochem. 2018. PMID: 30030363 Free PMC article. Review.
Cited by
- Two Novel Pathogenic Variants Confirm RMND1 Causative Role in Perrault Syndrome with Renal Involvement.
Oziębło D, Pazik J, Stępniak I, Skarżyński H, Ołdak M. Oziębło D, et al. Genes (Basel). 2020 Sep 8;11(9):1060. doi: 10.3390/genes11091060. Genes (Basel). 2020. PMID: 32911714 Free PMC article. - Evidence that 6q25.1 variant rs6931104 confers susceptibility to chronic myeloid leukemia through RMND1 regulation.
Woo YM, Kim S, Park JH, Lee NY, Kim JW, Kim DDH. Woo YM, et al. PLoS One. 2019 Jun 25;14(6):e0218968. doi: 10.1371/journal.pone.0218968. eCollection 2019. PLoS One. 2019. PMID: 31237926 Free PMC article. - Mitochondrial DNA transcription and translation: clinical syndromes.
Boczonadi V, Ricci G, Horvath R. Boczonadi V, et al. Essays Biochem. 2018 Jul 20;62(3):321-340. doi: 10.1042/EBC20170103. Print 2018 Jul 20. Essays Biochem. 2018. PMID: 29980628 Free PMC article. Review. - Failure to Guard: Mitochondrial Protein Quality Control in Cancer.
Friedlander JE, Shen N, Zeng A, Korm S, Feng H. Friedlander JE, et al. Int J Mol Sci. 2021 Aug 2;22(15):8306. doi: 10.3390/ijms22158306. Int J Mol Sci. 2021. PMID: 34361072 Free PMC article. Review. - The clinical maze of mitochondrial neurology.
DiMauro S, Schon EA, Carelli V, Hirano M. DiMauro S, et al. Nat Rev Neurol. 2013 Aug;9(8):429-44. doi: 10.1038/nrneurol.2013.126. Epub 2013 Jul 9. Nat Rev Neurol. 2013. PMID: 23835535 Free PMC article. Review.
References
- Koopman W.J., Willems P.H., Smeitink J.A. Monogenic mitochondrial disorders. N. Engl. J. Med. 2012;366:1132–1141. - PubMed
- Greaves L.C., Reeve A.K., Taylor R.W., Turnbull D.M. Mitochondrial DNA and disease. J. Pathol. 2012;226:274–286. - PubMed
- Yao J., Shoubridge E.A. Expression and functional analysis of SURF1 in Leigh syndrome patients with cytochrome c oxidase deficiency. Hum. Mol. Genet. 1999;8:2541–2549. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases