Movies of ice-embedded particles enhance resolution in electron cryo-microscopy - PubMed (original) (raw)
Movies of ice-embedded particles enhance resolution in electron cryo-microscopy
Melody G Campbell et al. Structure. 2012.
Abstract
Low-dose images obtained by electron cryo-microscopy (cryo-EM) are often affected by blurring caused by sample motion during electron beam exposure, degrading signal especially at high resolution. We show here that we can align frames of movies, recorded with a direct electron detector during beam exposure of rotavirus double-layered particles, thereby greatly reducing image blurring caused by beam-induced motion and sample stage instabilities. This procedure increases the efficiency of cryo-EM imaging and enhances the resolution obtained in three-dimensional reconstructions of the particle. Using movies in this way is generally applicable to all cryo-EM samples and should also improve the performance of midrange electron microscopes that may have limited mechanical stability and beam coherence.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure 1
Reduced image blurring with movie frame alignment. (A) An example of an image that experienced blurring due to a sample shift of about 10 Å. The white vectors indicate direction and amount of particle rotations measured between the first and last four frames of the recorded 16-frame movie. (B) The same image as in (A) after frame alignment based on the shifts measured for the DLP highlighted by the arrow. The white vectors indicate direction and amount of particle shifts measured between the first and last four frames of the movie. Additionally, shifts of the carbon support film were measured in four different locations using correlation maps between patches of 512 × 512 pixels of the four-frame averages. (C) Thon ring pattern (right) calculated from the image in (A) showing loss of contrast (arrow indicates the weaker or missing Thon rings) in one direction due to the sample motion, and fitted pattern (left) using the computer program CTFFIND3 (Mindell and Grigorieff, 2003). The fit extends to a resolution of 5 A and corresponds to a defocus of 1.1 μm. (D) Thon ring pattern and fit after frame alignment (image shown in (B)). The extension of the rings is now nearly isotropic (indicated by the arrow).
Figure 2
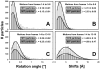
Histograms of the particle rotations and shifts measured by motion tracking using movies. There is no significant difference in the distributions of the whole data set (1,915 particles, light gray) and the best 42% of the data (807 particles, dark gray). Particle motions are measured between averages of frames 1 – 4 and 5 – 8 (A, B), and between 1 – 4 and 13 – 16 (C, D). Each distribution is fitted with a Rayleigh distribution to determine its maximum λ (the median motion value).
Figure 3
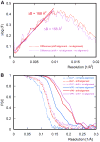
Improvement in resolution with frame alignment. (A) The difference between the natural logarithm of the average structure factors F (dotted lines) of DLP reconstructions obtained with and without frame alignment, as a function of squared reciprocal resolution. The plots allow estimation by a linear fit of the B-factor reduction obtained with shift alignment (red line) and additional rotational alignment (purple line). (B) FSC curves for reconstructions of icosahedrally averaged reconstructions of DLP and rotavirus VP6 coat protein after additional 13-fold non-icosahedral averaging, calculated using data from all 16 movie frames. The dotted and solid lines correspond to DLP and VP6 reconstructions, respectively, while blue, red and purple correspond to reconstructions obtained without frame alignment, with shift alignment, and with both shift and rotational alignment, respectively.
Figure 4
Comparison of density maps. (A) α-helical region of the VP6 density calculated using images derived from movies by simple frame averaging without motion tracking. Density for some of the larger amino acid side chains is visible. (B) Same as in (A) but with shift alignment of the movie frames to reduce blurring in the images. The side-chain density is significantly stronger compared to (A). (C) Same as in (A) but with additional rotational alignment of the movie frames taken into account during 3D reconstruction. (D) Same as in (A) for the map published in 2008 (EMDB 1461). The crystal structure of VP6 (PDB ID: 3KZ4, McClain et al., 2010) is superimposed on the density in (A) – (D) to aid interpretation of the features. For accurate comparison, the maps in (A), (B) and (C) were scaled to match the rotationally averaged amplitude spectrum of the map in (D) and contoured at the same density threshold as in (D) using UCSF Chimera (Goddard et al., 2007).
Similar articles
- Alignment of cryo-EM movies of individual particles by optimization of image translations.
Rubinstein JL, Brubaker MA. Rubinstein JL, et al. J Struct Biol. 2015 Nov;192(2):188-95. doi: 10.1016/j.jsb.2015.08.007. Epub 2015 Aug 19. J Struct Biol. 2015. PMID: 26296328 - Measuring the optimal exposure for single particle cryo-EM using a 2.6 Å reconstruction of rotavirus VP6.
Grant T, Grigorieff N. Grant T, et al. Elife. 2015 May 29;4:e06980. doi: 10.7554/eLife.06980. Elife. 2015. PMID: 26023829 Free PMC article. - Processing of Cryo-EM Movie Data.
Ripstein ZA, Rubinstein JL. Ripstein ZA, et al. Methods Enzymol. 2016;579:103-24. doi: 10.1016/bs.mie.2016.04.009. Epub 2016 Jun 1. Methods Enzymol. 2016. PMID: 27572725 Review. - Beam-induced motion of vitrified specimen on holey carbon film.
Brilot AF, Chen JZ, Cheng A, Pan J, Harrison SC, Potter CS, Carragher B, Henderson R, Grigorieff N. Brilot AF, et al. J Struct Biol. 2012 Mar;177(3):630-7. doi: 10.1016/j.jsb.2012.02.003. Epub 2012 Feb 16. J Struct Biol. 2012. PMID: 22366277 Free PMC article. - Single-particle cryo-EM data acquisition by using direct electron detection camera.
Wu S, Armache JP, Cheng Y. Wu S, et al. Microscopy (Oxf). 2016 Feb;65(1):35-41. doi: 10.1093/jmicro/dfv355. Epub 2015 Nov 6. Microscopy (Oxf). 2016. PMID: 26546989 Free PMC article. Review.
Cited by
- Cryo-EM phase-plate images reveal unexpected levels of apparent specimen damage.
Remis J, Petrov PN, Zhang JT, Axelrod JJ, Cheng H, Sandhaus S, Mueller H, Glaeser RM. Remis J, et al. bioRxiv [Preprint]. 2024 Aug 6:2024.08.04.606536. doi: 10.1101/2024.08.04.606536. bioRxiv. 2024. PMID: 39149370 Free PMC article. Preprint. - Use of phase plate cryo-EM reveals conformation diversity of therapeutic IgG with 50 kDa Fab fragment resolved below 6 Å.
Lin HH, Wang CH, Huang SH, Lin SY, Kato T, Namba K, Hosogi N, Song C, Murata K, Yen CH, Hsu TL, Wong CH, Wu YM, Tu IP, Chang WH. Lin HH, et al. Sci Rep. 2024 Jun 18;14(1):14079. doi: 10.1038/s41598-024-62045-8. Sci Rep. 2024. PMID: 38890341 Free PMC article. - Bridging structural and cell biology with cryo-electron microscopy.
Nogales E, Mahamid J. Nogales E, et al. Nature. 2024 Apr;628(8006):47-56. doi: 10.1038/s41586-024-07198-2. Epub 2024 Apr 3. Nature. 2024. PMID: 38570716 Free PMC article. Review. - Overcoming resolution attenuation during tilted cryo-EM data collection.
Aiyer S, Baldwin PR, Tan SM, Shan Z, Oh J, Mehrani A, Bowman ME, Louie G, Passos DO, Đorđević-Marquardt S, Mietzsch M, Hull JA, Hoshika S, Barad BA, Grotjahn DA, McKenna R, Agbandje-McKenna M, Benner SA, Noel JAP, Wang D, Tan YZ, Lyumkis D. Aiyer S, et al. Nat Commun. 2024 Jan 9;15(1):389. doi: 10.1038/s41467-023-44555-7. Nat Commun. 2024. PMID: 38195598 Free PMC article. - A minority of final stacks yields superior amplitude in single-particle cryo-EM.
Zhu J, Zhang Q, Zhang H, Shi Z, Hu M, Bao C. Zhu J, et al. Nat Commun. 2023 Dec 10;14(1):7822. doi: 10.1038/s41467-023-43555-x. Nat Commun. 2023. PMID: 38072910 Free PMC article.
References
- Baker LA, Smith EA, Bueler SA, Rubinstein JL. The resolution dependence of optimal exposures in liquid nitrogen temperature electron cryomicroscopy of catalase crystals. J Struct Biol. 2010;169:431–437. - PubMed
- Bottcher B, Wynne SA, Crowther RA. Determination of the fold of the core protein of hepatitis B virus by electron cryomicroscopy. Nature. 1997;386:88–91. - PubMed
- Bullough P, Henderson R. Use of spot-scan procedure for recording low-dose micrographs of beam-sensitive specimens. Ultramicroscopy. 1987;21:223–229.
- Cambie R, Downing KH, Typke D, Glaeser RM, Jin J. Design of a microfabricated, two-electrode phase-contrast element suitable for electron microscopy. Ultramicroscopy. 2007;107:329–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 GM062580/GM/NIGMS NIH HHS/United States
- RR017573/RR/NCRR NIH HHS/United States
- 9 P41 GM103310-11/GM/NIGMS NIH HHS/United States
- P01 GM62580/GM/NIGMS NIH HHS/United States
- P41 GM103310/GM/NIGMS NIH HHS/United States
- P41 RR017573/RR/NCRR NIH HHS/United States
- 2P41RR017573-11/RR/NCRR NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources