Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm - PubMed (original) (raw)
. 2012 Nov;44(11):1249-54.
doi: 10.1038/ng.2421. Epub 2012 Sep 30.
Jefferson J Doyle, Seneca L Bessling, Samantha Maragh, Mark E Lindsay, Dorien Schepers, Elisabeth Gillis, Geert Mortier, Tessa Homfray, Kimberly Sauls, Russell A Norris, Nicholas D Huso, Dan Leahy, David W Mohr, Mark J Caulfield, Alan F Scott, Anne Destrée, Raoul C Hennekam, Pamela H Arn, Cynthia J Curry, Lut Van Laer, Andrew S McCallion, Bart L Loeys, Harry C Dietz
Affiliations
- PMID: 23023332
- PMCID: PMC3545695
- DOI: 10.1038/ng.2421
Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm
Alexander J Doyle et al. Nat Genet. 2012 Nov.
Abstract
Elevated transforming growth factor (TGF)-β signaling has been implicated in the pathogenesis of syndromic presentations of aortic aneurysm, including Marfan syndrome (MFS) and Loeys-Dietz syndrome (LDS). However, the location and character of many of the causal mutations in LDS intuitively imply diminished TGF-β signaling. Taken together, these data have engendered controversy regarding the specific role of TGF-β in disease pathogenesis. Shprintzen-Goldberg syndrome (SGS) has considerable phenotypic overlap with MFS and LDS, including aortic aneurysm. We identified causative variation in ten individuals with SGS in the proto-oncogene SKI, a known repressor of TGF-β activity. Cultured dermal fibroblasts from affected individuals showed enhanced activation of TGF-β signaling cascades and higher expression of TGF-β-responsive genes relative to control cells. Morpholino-induced silencing of SKI paralogs in zebrafish recapitulated abnormalities seen in humans with SGS. These data support the conclusions that increased TGF-β signaling is the mechanism underlying SGS and that high signaling contributes to multiple syndromic presentations of aortic aneurysm.
Figures
Figure 1
SKI mutations in patients with Shprintzen-Goldberg syndrome (SGS). A) Clinical features and mutations seen in SGS patients. Illustrated features involve the craniofacial (abnormal head shape due to craniosynostosis, widely-spaced eyes, small and receding chin and high-arched palate), skeletal (long fingers, joint contractures, chest wall deformity, spine curvature, foot deformity) and cardiovascular (aortic root aneurysm indicated by black arrowheads and mitral valve prolapse indicated by white arrowhead) systems. Pedigrees indicate that all cases were sporadic (affected, black symbols; unaffected, open symbols). Mutation status is indicated below each individual (−/−, mutation negative; +/−, heterozygous; blank, not available for testing). Permission to publish photographs was obtained from the affected individuals or their parents. B) Location of mutations in relation to known binding sites (highlighted in yellow) of SKI binding partners. The position of the Dachshund homology domain is indicated.
Figure 2
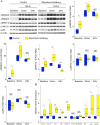
Assessment of TGF-β signaling in dermal fibroblasts. A) Western blot analysis of pSMAD2, pSMAD3, pERK1/2, pJNK1/2, pp38 and β-Actin (loading control) at steady state (baseline) and in response to TGF-β2. Representative Shprintzen-Goldberg syndrome (SGS) and control blots are shown (for a single SGS patient and single control with 3 biological replicates for each). Graphs display Western blot quantification of 3 biological replicates in each of 2 SGS patients and 2 controls. All data are normalized to β-Actin. The control baseline value for each graph was set to 1.0; all other values represent fold change in comparison to this sample. B) Relative mRNA expression for TGF-β target genes (normalized to β-Actin), as assessed by quantitative polymerase chain reaction analysis. Quantification was performed on 3 biological replicates in each of 2 SGS patients and 2 controls. The upper and lower margins of the box define the 75th and 25th percentiles respectively; the internal line defines the median and the whiskers define the range. NS, not significant; * p<0.05; ** p<0.01; † p<0.001; †† p<0.0001.
Figure 3
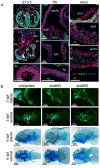
Assessment of SKI in model systems. A) Murine SKI expression at embryonic day 13.5 (E13.5) is high in the mitral valve (MV), tricupsid valve (TV), pulmonary trunk (Pu), pulmonary valve (PuV), bronchus (Br) and proximal aorta (Ao; both nucleus and cytoplasm). Lower expression is seen in the descending aorta (dAo), esophagus (E), right and left ventricles (RV and LV) and left atrium (LA). Red, SKI; green, myosin heavy chain; blue, nuclei. At birth (P0), SKI is expressed in the aortic root (AoR), distal ascending aorta (ascAo) and endothelial surface of the mitral and aortic (AoV) valves, with less nuclear expression than at E13.5. In adulthood, SKI is expressed exclusively in the cytoplasm of the AoV and the media of the AoR; in the ascAo SKI is expressed in the intima (I) and adventitia (A), but excluded from the central media (M). B) Disruption of skia or skib expression in zebrafish. Top panels, assessment of cardiovascular anatomy at 3 days post-fertilization (dpf). Uninjected embryos display proper cardiac looping with normal relation between the atrium (A, dotted outline) and ventricle (V), and a distinct bulbous arteriosus (arrow) defining the outflow tract (OFT). skia morphants show incomplete looping with an irregular OFT, while skib morphants show failure of looping and an ill-defined OFT. Asterisk, atrioventricular cushion. Lower panels, alcian blue staining of cartilage at 6 dpf. skia and skib morphants display craniofacial cartilage deficits and prominent mandibular malformation. Meckel's cartilage, m; palatoquadrates, pq; ceratohyales, ch; ceratobranchial arches, cb. Scale bars, μm.
Comment in
- Aberrant TGF-β signaling underlies the pathogenesis of aortic aneurysm in Shprintzen-Goldberg syndrome.
Jan A. Jan A. Clin Genet. 2013 Apr;83(4):318-9. doi: 10.1111/cge.12102. Clin Genet. 2013. PMID: 23330586 No abstract available.
Similar articles
- Mutations in SKI in Shprintzen-Goldberg syndrome lead to attenuated TGF-β responses through SKI stabilization.
Gori I, George R, Purkiss AG, Strohbuecker S, Randall RA, Ogrodowicz R, Carmignac V, Faivre L, Joshi D, Kjær S, Hill CS. Gori I, et al. Elife. 2021 Jan 8;10:e63545. doi: 10.7554/eLife.63545. Elife. 2021. PMID: 33416497 Free PMC article. - Marfan Syndrome and Related Disorders: 25 Years of Gene Discovery.
Verstraeten A, Alaerts M, Van Laer L, Loeys B. Verstraeten A, et al. Hum Mutat. 2016 Jun;37(6):524-31. doi: 10.1002/humu.22977. Epub 2016 Mar 14. Hum Mutat. 2016. PMID: 26919284 Review. - De novo exon 1 missense mutations of SKI and Shprintzen-Goldberg syndrome: two new cases and a clinical review.
Au PY, Racher HE, Graham JM Jr, Kramer N, Lowry RB, Parboosingh JS, Innes AM; FORGE Canada Consortium. Au PY, et al. Am J Med Genet A. 2014 Mar;164A(3):676-84. doi: 10.1002/ajmg.a.36340. Epub 2013 Dec 19. Am J Med Genet A. 2014. PMID: 24357594 Review. - Shprintzen - Goldberg syndrome without intellectual disability: A clinical report and review of literature.
Chatelain C, Kukor L, Bailleux S, Bours V, Bulk S, Docampo E. Chatelain C, et al. Eur J Med Genet. 2025 Feb;73:104985. doi: 10.1016/j.ejmg.2024.104985. Epub 2024 Dec 3. Eur J Med Genet. 2025. PMID: 39638120 Review. - The SMAD-binding domain of SKI: a hotspot for de novo mutations causing Shprintzen-Goldberg syndrome.
Schepers D, Doyle AJ, Oswald G, Sparks E, Myers L, Willems PJ, Mansour S, Simpson MA, Frysira H, Maat-Kievit A, Van Minkelen R, Hoogeboom JM, Mortier GR, Titheradge H, Brueton L, Starr L, Stark Z, Ockeloen C, Lourenco CM, Blair E, Hobson E, Hurst J, Maystadt I, Destrée A, Girisha KM, Miller M, Dietz HC, Loeys B, Van Laer L. Schepers D, et al. Eur J Hum Genet. 2015 Feb;23(2):224-8. doi: 10.1038/ejhg.2014.61. Epub 2014 Apr 16. Eur J Hum Genet. 2015. PMID: 24736733 Free PMC article.
Cited by
- Genes responsible for proliferation, differentiation, and junction adhesion are significantly up-regulated in human ovarian granulosa cells during a long-term primary in vitro culture.
Kranc W, Brązert M, Budna J, Celichowski P, Bryja A, Nawrocki MJ, Ożegowska K, Jankowski M, Chermuła B, Dyszkiewicz-Konwińska M, Jeseta M, Pawelczyk L, Bręborowicz A, Rachoń D, Bruska M, Nowicki M, Zabel M, Kempisty B. Kranc W, et al. Histochem Cell Biol. 2019 Feb;151(2):125-143. doi: 10.1007/s00418-018-1750-1. Epub 2018 Oct 31. Histochem Cell Biol. 2019. PMID: 30382374 Free PMC article. - Tgfbr2 disruption in postnatal smooth muscle impairs aortic wall homeostasis.
Li W, Li Q, Jiao Y, Qin L, Ali R, Zhou J, Ferruzzi J, Kim RW, Geirsson A, Dietz HC, Offermanns S, Humphrey JD, Tellides G. Li W, et al. J Clin Invest. 2014 Feb;124(2):755-67. doi: 10.1172/JCI69942. Epub 2014 Jan 9. J Clin Invest. 2014. PMID: 24401272 Free PMC article. - Genes Associated with Thoracic Aortic Aneurysm and Dissection: An Update and Clinical Implications.
Brownstein AJ, Ziganshin BA, Kuivaniemi H, Body SC, Bale AE, Elefteriades JA. Brownstein AJ, et al. Aorta (Stamford). 2017 Feb 1;5(1):11-20. doi: 10.12945/j.aorta.2017.17.003. eCollection 2017 Feb. Aorta (Stamford). 2017. PMID: 28868310 Free PMC article. Review. - A human importin-β-related disorder: Syndromic thoracic aortic aneurysm caused by bi-allelic loss-of-function variants in IPO8.
Van Gucht I, Meester JAN, Bento JR, Bastiaansen M, Bastianen J, Luyckx I, Van Den Heuvel L, Neutel CHG, Guns PJ, Vermont M, Fransen E, Perik MHAM, Velchev JD, Alaerts M, Schepers D, Peeters S, Pintelon I, Almesned A, Ferla MP, Taylor JC, Dallosso AR, Williams M, Evans J; Genomics England Research Consortium; Rosenfeld JA, Sluysmans T, Rodrigues D, Chikermane A, Bharmappanavara G, Vijayakumar K, Mottaghi Moghaddam Shahri H, Hashemi N, Torbati PN, Toosi MB, Al-Hassnan ZN, Vogt J, Revencu N, Maystadt I, Miller EM, Weaver KN, Begtrup A, Houlden H, Murphy D, Maroofian R, Pagnamenta AT, Van Laer L, Loeys BL, Verstraeten A. Van Gucht I, et al. Am J Hum Genet. 2021 Jun 3;108(6):1115-1125. doi: 10.1016/j.ajhg.2021.04.019. Epub 2021 May 18. Am J Hum Genet. 2021. PMID: 34010605 Free PMC article. - Complications of Insufficient Dura and Blood Loss During Surgical Intervention in Shprintzen-Goldberg Syndrome: A Case Report.
O'Dougherty GR, Fulkerson DH, Kern M, Haldar K, Calhoun B. O'Dougherty GR, et al. Am J Case Rep. 2019 Aug 8;20:1159-1169. doi: 10.12659/AJCR.914924. Am J Case Rep. 2019. PMID: 31391415 Free PMC article.
References
- Dietz HC, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. - PubMed
- Loeys BL, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. - PubMed
- Loeys BL, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–798. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- G9521010/MRC_/Medical Research Council/United Kingdom
- T32 GM007814/GM/NIGMS NIH HHS/United States
- U54 HG006542/HG/NHGRI NIH HHS/United States
- R01 GM099321/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- 1R01HL111267/HL/NHLBI NIH HHS/United States
- 1U54HG006542/HG/NHGRI NIH HHS/United States
- K08 HL107738/HL/NHLBI NIH HHS/United States
- P01-AR049698/AR/NIAMS NIH HHS/United States
- G0600237/MRC_/Medical Research Council/United Kingdom
- R01 HL111267/HL/NHLBI NIH HHS/United States
- R01 AR041135/AR/NIAMS NIH HHS/United States
- P01 AR049698/AR/NIAMS NIH HHS/United States
- R01-AR41135/AR/NIAMS NIH HHS/United States
- K08 HL107738-01/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases