Autocrine prolactin induced by the Pten-Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways - PubMed (original) (raw)
Autocrine prolactin induced by the Pten-Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways
Chien-Chung Chen et al. Genes Dev. 2012.
Abstract
Extrapituitary prolactin (Prl) is produced in humans and rodents; however, little is known about its in vivo regulation or physiological function. We now report that autocrine prolactin is required for terminal mammary epithelial differentiation during pregnancy and that its production is regulated by the Pten-PI3K-Akt pathway. Conditional activation of the PI3K-Akt pathway in the mammary glands of virgin mice by either Akt1 expression or Pten deletion rapidly induced terminal mammary epithelial differentiation accompanied by the synthesis of milk despite the absence of lobuloalveolar development. Surprisingly, we found that mammary differentiation was due to the PI3K-Akt-dependent synthesis and secretion of autocrine prolactin and downstream activation of the prolactin receptor (Prlr)-Jak-Stat5 pathway. Consistent with this, Akt-induced mammary differentiation was abrogated in Prl(-/-), Prlr(-/-), and Stat5(-/-) mice. Furthermore, cells treated with conditioned medium from mammary glands in which Akt had been activated underwent rapid Stat5 phosphorylation in a manner that was blocked by inhibition of Jak2, treatment with an anti-Prl antibody, or deletion of the prolactin gene. Demonstrating a physiological requirement for autocrine prolactin, mammary glands from lactation-defective Akt1(-/-);Akt2(+/-) mice failed to express autocrine prolactin or activate Stat5 during late pregnancy despite normal levels of circulating serum prolactin and pituitary prolactin production. Our findings reveal that PI3K-Akt pathway activation is necessary and sufficient to induce autocrine prolactin production in the mammary gland, Stat5 activation, and terminal mammary epithelial differentiation, even in the absence of the normal developmental program that prepares the mammary gland for lactation. Together, these findings identify a function for autocrine prolactin during normal development and demonstrate its endogenous regulation by the PI3K-Akt pathway.
Figures
Figure 1.
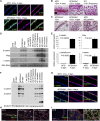
Akt activation induces secretory differentiation and milk production in the virgin mammary gland. (A) Immunofluorescence analysis for expression of p-Akt and the luminal epithelial marker cytokeratin 8 (CK8) in the mammary glands of MTB and MTB/tAkt1 mice induced with doxycycline for 96 h. Nuclei were counterstained with Hoechst 33258 (blue). Bars, 50 μm. (B) Whole-mount staining of the mammary glands of MTB and MTB/tAkt1 mice induced with doxycycline for 96 h. Bars, 5 mm. (C) H&E-stained sections of mammary tissue from induced (+Dox) and uninduced (−Dox) MTB/tAkt1 mice and lactating MTB control mice. Bars, 100 μm. (D) Northern analysis of milk protein gene expression in mammary glands from doxycycline-induced MTB and MTB/tAkt1 mice. Wild-type FVB mice at the indicated developmental stages are shown as controls for the temporal expression patterns of individual milk protein genes. 28S rRNA served as a loading control. (E) Quantitative RT–PCR analysis of milk protein gene expression in mammary glands from induced MTB and MTB/tAkt1 mice. Gene expression levels were normalized to cytokeratin 18. Error bars represent mean ± standard error of the mean (SEM). (F) Immunoblotting analysis of total milk proteins in mammary glands from MTB and MTB/tAkt1 mice induced with doxycycline for 96 h and wild-type FVB mice at the indicated developmental stages. β-Tubulin served as a loading control. (G) Immunofluorescence analysis of β-casein and CK8 expression in the mammary glands of MTB and MTB/tAkt1 mice induced with doxycycline for 96 h. Nuclei were counterstained with Hoechst 33258 (blue). Bars, 50 μm. (H,I) Immunofluorescence analysis of NKCC1 (H) and Npt2b (I) expression in the mammary glands of MTB and MTB/tAkt1 mice induced with doxycycline for 96 h as well as lactating wild-type controls. Arrows indicate epithelial cells that have down-regulated NKCC1 expression. Nuclei were counterstained with Hoechst 33258 (blue). Bars, 100 μm.
Figure 2.
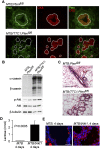
Pten deletion induces secretory differentiation in virgin mice. (A) Immunofluorescence analysis for expression of Pten and the luminal epithelial marker CK8 in the mammary glands of virgin MTB;Ptenfl/fl and MTB/TTC1;Ptenfl/fl mice induced with doxycycline for 2 wk. Bars, 50 μm. Dotted lines indicate the demarcation between the mammary epithelium and the mammary stroma. (B) Immunoblotting analysis of mammary protein lysates from MTB;Ptenfl/fl and MTB/TTC1;Ptenfl/fl mice induced with doxycycline for 2 wk. β-Tubulin served as a loading control. (C) H&E-stained sections of mammary tissues from B. Bars, 100 μm. (D) Lactose levels of mammary tissues from induced MTB and MTB/tAkt1 mice (n = 4). Error bars indicate mean ± SEM. (E) Nile red staining of cytoplasmic lipid droplets in the mammary glands of MTB and MTB/tAkt1 mice induced with doxycycline for 96 h. Nuclei were counterstained with Hoechst 33258 (blue). Bars, 50 μm.
Figure 3.
Akt induces Stat5 activation. (A) Immunoblotting analysis of mammary protein lysates from virgin MTB and MTB/tAkt1 mice induced with doxycycline for 96 h. The top bands of the p-Akt and Akt doublets correspond to the transgene-encoded myristoylated Akt1. β-Tubulin served as a loading control. (B) Immunoblotting analysis of mammary protein lysates from virgin MTB;Ptenfl/fl and MTB/TTC1;Ptenfl/fl mice induced with doxycycline for 14 d. β-Tubulin served as a loading control. (C) Immunofluorescence analysis of p-Stat5a/b and p-Akt expression in mammary sections from virgin MTB and MTB/tAkt1 mice induced with doxycycline for 24 or 72 h. Bars, 50 μm. (D) Immunofluorescence analysis of p-Stat5a/b and p-Akt expression in mammary sections from virgin MTB;Ptenfl/fl and MTB/TTC1;Ptenfl/fl mice induced with doxycycline for 14 d. Bars, 50 μm.
Figure 4.
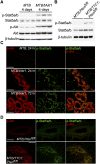
Akt-mediated differentiation of the virgin mammary gland requires Stat5a/b and Prlr. (A) H&E-stained mammary sections from doxycycline-induced virgin MTB and MTB/tAkt1 wild-type mice or mice heterozygous or homozygous for a hypomorphic allele of Stat5a/b. Bars, 200 μm. (B) Immunoblotting analysis of mammary protein lysates corresponding to mice in A. The faster-migrating p-Stat5a/b and Stat5a/b bands represent the N-terminal-truncated Stat5a/b protein encoded by the hypomorphic allele of Stat5a/b. β-Tubulin served as a loading control. (C) Immunofluorescence analysis of NKCC1 (top) and Npt2b (bottom) expression in mammary tissues from doxycycline-induced 6-wk-old virgin MTB/tAkt1 mice of the indicated Stat5a/b genotypes. Luminal epithelial cells were coimmunostained with CK8. Arrows denote mammary epithelial cells in which NKCC1 expression was not detected. Mammary tissues from MTB mice homozygous for mutant alleles of Stat5a/b served as negative controls. Nuclei were counterstained with Hoechst 33258 (blue). Bars, 100 μm. (D) H&E-stained mammary sections from doxycycline-induced virgin MTB/tAkt1 wild-type mice and mice heterozygous or homozygous for a null allele of Prlr. Bars, 200 μm. (E) Immunoblotting analysis of mammary protein lysates corresponding to mice in D. (F) Immunofluorescence analysis of NKCC1 (top) and Npt2b (bottom) expression in mammary tissues from doxycycline-induced 6-wk-old virgin MTB/tAkt1 mice of the indicated Prlr genotypes. Luminal epithelial cells were coimmunostained with CK8. Arrows denote mammary epithelial cells in which NKCC1 expression was not detected. Mammary tissues from MTB mice homozygous for mutant alleles of Prlr served as negative controls. Nuclei were counterstained with Hoechst 33258 (blue). Bars, 100 μm. (G) Nile red staining of cytoplasmic lipid droplets in the mammary glands of doxycycline-induced virgin MTB/tAkt1, MTB/tAkt1;Stat5a/b−/−, and MTB/tAkt1;Prlr−/− mice. Bars, 50 μm. (H) Lactose levels in mammary glands from doxycycline-induced virgin MTB/tAkt1, MTB/tAkt1;Stat5a/b−/−, and MTB/tAkt1;Prlr−/− mice. _MTB;Stat5a/b_−/− and MTB;Prlr−/− mice are included as controls. Error bars indicate mean ± SEM.
Figure 5.
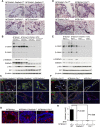
Akt induces expression of a secreted factor responsible for Stat5 activation and milk protein expression. (A) Immunoblotting analysis of protein lysates from MTB/tAkt1 mammary glands treated ex vivo with the indicated doses of prolactin in the absence (−Dox) or presence (+Dox) of 2 μg/mL doxycycline. (B) Immunoblotting analysis of Stat5a/b immunoprecipitated from HC11 cells incubated for 20 min with CM harvested from wild-type MTB/tAkt1 mammary tissues induced with doxycycline for 4 d ex vivo (+Dox) or untreated (−Dox). Lysate from HC11 cells treated with 1 μg/mL prolactin is shown as a positive control. (C) Immunoblotting analysis of Stat5a/b immunoprecipitated from HC11 cells incubated for 20 min with CM harvested from wild-type or Stat5a/b−/− MTB/tAkt1 mammary tissues induced with doxycycline ex vivo. (D) Immunoblotting analysis of Stat5a/b immunoprecipitated from HC11 cells incubated for 20 min with CM harvested from wild-type or Prlr−/− MTB/tAkt1 mammary tissues induced with doxycycline ex vivo.
Figure 6.
Akt induces autocrine prolactin expression in the mammary gland. (A) Immunoblotting analysis of prolactin expression in the mammary glands of virgin MTB and MTB/tAkt1 mice induced with doxycycline for 96 h. Protein lysates from pituitary glands of wild-type mice served as a positive control. β-Tubulin served as a loading control. (B) Immunoblotting analysis of prolactin expression in the mammary glands of MTB;Ptenfl/fl and MTB/TTC1;Ptenfl/fl mice induced with doxycycline for 4 wk. β-Tubulin served as a loading control. (C) Immunoblotting analysis of prolactin expression in purified epithelial and adipose fractions generated from the mammary glands of MTB and MTB/tAkt1 mice treated with doxycycline for 4 d. E-cadherin and adiponectin served as controls for epithelial and adipose cells, respectively. β-Actin served as a loading control. (D) Northern analysis of prolactin mRNA expression in mammary glands from doxycycline-induced virgin MTB and MTB/tAkt1 mice. 18S rRNA served as an RNA loading control. (E) Serum prolactin levels in MTB and MTB/tAkt1 mice induced with doxycycline for 96 h (n = 5). (F) Immunoblotting analysis of Stat5a/b immunoprecipitates from HC11 cells incubated for 20 min with CM harvested from wild-type MTB/tAkt1 mammary tissues induced with doxycycline for 4 d ex vivo (+Dox) or untreated (−Dox). CM were preincubated with anti-prolactin antibody or IgG control for 30 min at room temperature prior to incubation with HC11 cells. Error bars represent mean ± SEM. (G) Immunoblotting analysis of Stat5a/b immunoprecipitated from HC11 cells pretreated with the Jak2 inhibitor NVP-BSK805 (1 μM) for 1 h followed by incubation with CM for 20 min. CM was harvested from wild-type MTB/tAkt1 mammary tissues induced with doxycycline for 4 d ex vivo (+Dox) or untreated (−Dox).
Figure 7.
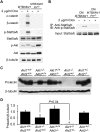
Prl is required for Akt1-mediated Stat5 activation and mammary differentiation. (A) Immunoblotting analysis of the indicated proteins in protein lysates from virgin MTB/tAkt1 and MTB/tAkt1;Prl−/− mammary tissues induced with doxycycline for 4 d ex vivo (+Dox) or untreated (−Dox). β-Tubulin levels served as a loading control. (B) Immunoblotting analysis of Stat5a/b immunoprecipitated from HC11 cells treated for 20 min with CM from MTB/tAkt1 and MTB/tAkt1;Prl−/− mammary tissues induced with doxycycline ex vivo. (C) Immunoblotting analysis of prolactin expression in the mammary glands of mice bearing the indicated Akt genotypes at day 18.5 of pregnancy. β-Tubulin served as a loading control. (D) Quantification of prolactin expression in the mammary glands of mice bearing the indicated Akt1 and Akt2 genotypes at day 18.5 of pregnancy (n = 6 per genotype) normalized to β-tubulin expression. Prolactin/β-tubulin ratios were normalized to those in wild-type mice. Error bars indicate mean ± SEM. Akt1−/−;Akt2+/− vs. wild type, (*) P < 0.001; Akt1−/−;Akt2+/− vs. Akt1−/−;Akt2+/+, P = 0.04, as indicated.
Comment in
- Autocrine prolactin: an emerging market for homegrown (prolactin) despite the imports.
Muthuswamy SK. Muthuswamy SK. Genes Dev. 2012 Oct 15;26(20):2253-8. doi: 10.1101/gad.204636.112. Genes Dev. 2012. PMID: 23070811 Free PMC article.
Similar articles
- Akt is required for Stat5 activation and mammary differentiation.
Chen CC, Boxer RB, Stairs DB, Portocarrero CP, Horton RH, Alvarez JV, Birnbaum MJ, Chodosh LA. Chen CC, et al. Breast Cancer Res. 2010;12(5):R72. doi: 10.1186/bcr2640. Epub 2010 Sep 17. Breast Cancer Res. 2010. PMID: 20849614 Free PMC article. - ErbB3 drives mammary epithelial survival and differentiation during pregnancy and lactation.
Williams MM, Vaught DB, Joly MM, Hicks DJ, Sanchez V, Owens P, Rahman B, Elion DL, Balko JM, Cook RS. Williams MM, et al. Breast Cancer Res. 2017 Sep 8;19(1):105. doi: 10.1186/s13058-017-0893-7. Breast Cancer Res. 2017. PMID: 28886748 Free PMC article. - Prolactin regulates ZNT2 expression through the JAK2/STAT5 signaling pathway in mammary cells.
Qian L, Lopez V, Seo YA, Kelleher SL. Qian L, et al. Am J Physiol Cell Physiol. 2009 Aug;297(2):C369-77. doi: 10.1152/ajpcell.00589.2008. Epub 2009 Jun 3. Am J Physiol Cell Physiol. 2009. PMID: 19494234 Free PMC article. - STAT5-Driven Enhancers Tightly Control Temporal Expression of Mammary-Specific Genes.
Shin HY, Hennighausen L, Yoo KH. Shin HY, et al. J Mammary Gland Biol Neoplasia. 2019 Mar;24(1):61-71. doi: 10.1007/s10911-018-9418-y. Epub 2018 Oct 17. J Mammary Gland Biol Neoplasia. 2019. PMID: 30328555 Review. - Triennial Lactation Symposium: Prolactin: The multifaceted potentiator of mammary growth and function.
Trott JF, Schennink A, Petrie WK, Manjarin R, VanKlompenberg MK, Hovey RC. Trott JF, et al. J Anim Sci. 2012 May;90(5):1674-86. doi: 10.2527/jas.2011-4682. Epub 2011 Dec 28. J Anim Sci. 2012. PMID: 22205663 Review.
Cited by
- An interpretive review of selective sweep studies in Bos taurus cattle populations: identification of unique and shared selection signals across breeds.
Gutiérrez-Gil B, Arranz JJ, Wiener P. Gutiérrez-Gil B, et al. Front Genet. 2015 May 13;6:167. doi: 10.3389/fgene.2015.00167. eCollection 2015. Front Genet. 2015. PMID: 26029239 Free PMC article. Review. - New insights in prolactin: pathological implications.
Bernard V, Young J, Chanson P, Binart N. Bernard V, et al. Nat Rev Endocrinol. 2015 May;11(5):265-75. doi: 10.1038/nrendo.2015.36. Epub 2015 Mar 17. Nat Rev Endocrinol. 2015. PMID: 25781857 Review. - Completely humanizing prolactin rescues infertility in prolactin knockout mice and leads to human prolactin expression in extrapituitary mouse tissues.
Christensen HR, Murawsky MK, Horseman ND, Willson TA, Gregerson KA. Christensen HR, et al. Endocrinology. 2013 Dec;154(12):4777-89. doi: 10.1210/en.2013-1476. Epub 2013 Sep 12. Endocrinology. 2013. PMID: 24029242 Free PMC article. - The JAK-STAT transcriptional regulator, STAT-5, activates the ATM DNA damage pathway to induce HPV 31 genome amplification upon epithelial differentiation.
Hong S, Laimins LA. Hong S, et al. PLoS Pathog. 2013;9(4):e1003295. doi: 10.1371/journal.ppat.1003295. Epub 2013 Apr 4. PLoS Pathog. 2013. PMID: 23593005 Free PMC article. - Effects of Dietary Supplementation of Lauric Acid on Lactation Function, Mammary Gland Development, and Serum Lipid Metabolites in Lactating Mice.
Yang L, Yang Q, Li F, Yi W, Liu F, Wang S, Jiang Q. Yang L, et al. Animals (Basel). 2020 Mar 22;10(3):529. doi: 10.3390/ani10030529. Animals (Basel). 2020. PMID: 32235692 Free PMC article.
References
- Acosta JJ, Munoz RM, Gonzalez L, Subtil-Rodriguez A, Dominguez-Caceres MA, Garcia-Martinez JM, Calcabrini A, Lazaro-Trueba I, Martin-Perez J 2003. Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol Endocrinol 17: 2268–2282 - PubMed
- Altomare DA, Testa JR 2005. Perturbations of the AKT signaling pathway in human cancer. Oncogene 24: 7455–7464 - PubMed
- Baffert F, Regnier CH, De Pover A, Pissot-Soldermann C, Tavares GA, Blasco F, Brueggen J, Chene P, Drueckes P, Erdmann D, et al. 2010. Potent and selective inhibition of polycythemia by the quinoxaline JAK2 inhibitor NVP-BSK805. Mol Cancer Ther 9: 1945–1955 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous