Slc15a4, a gene required for pDC sensing of TLR ligands, is required to control persistent viral infection - PubMed (original) (raw)
Slc15a4, a gene required for pDC sensing of TLR ligands, is required to control persistent viral infection
Amanda L Blasius et al. PLoS Pathog. 2012 Sep.
Abstract
Plasmacytoid dendritic cells (pDCs) are the major producers of type I IFN in response to viral infection and have been shown to direct both innate and adaptive immune responses in vitro. However, in vivo evidence for their role in viral infection is lacking. We evaluated the contribution of pDCs to acute and chronic virus infection using the feeble mouse model of pDC functional deficiency. We have previously demonstrated that feeble mice have a defect in TLR ligand sensing. Although pDCs were found to influence early cytokine secretion, they were not required for control of viremia in the acute phase of the infection. However, T cell priming was deficient in the absence of functional pDCs and the virus-specific immune response was hampered. Ultimately, infection persisted in feeble mice. We conclude that pDCs are likely required for efficient T cell priming and subsequent viral clearance. Our data suggest that reduced pDC functionality may lead to chronic infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Slc15a4 is required to control chronic viral infection.
Serum and organs were collected at the indicated times post LCMV infection and infectious virus was titered. WT and feeble mice were infected i.v. with 2×106 pfu of the acute LCMV Armstrong (Arm) strain or the persistent LCMV Clone 13 (Cl13) strain (A). Serum was then harvested at the indicated time points for >4 months to enumerate viremia. After Cl13 infection, organs were harvested from WT and feeble mice 5 dpi (B), 8 dpi (C), and 2 mpi (D), homogenized and titered for infectious virus. Individual replicates and means are shown. Feeble, Slc15a4feeble/feeble mice; l.o.d., limit of detection. Representative data of 2 independent experiments are shown; n> = 5 per group. Unless marked, p>0.05 between WT and feeble and not statistically significantly different.
Figure 2. Slc15a4 is required for antigen specific T cell responses in vivo.
8 days after LCMV Cl13 infection, splenocytes from WT and feeble mice were cultured ex vivo with the indicated peptides at 10−7 M, 10−8 M (“lo” concentration) or without peptide for 5 hours and then stained with antibodies to quantitate T cell antigen specific production of IFN-γ (A) and TNF-α (B). In (C) mice were primed with TAP1−/− 5E1 fibroblasts expressing the model antigen from Adeno E1B. Seven days after i.p. injection splenocytes from WT, feeble, and inept mice were cultured ex vivo with the immunodominant antigen for 5 hours. Then IFN-γ expression was quantitated in CD8+ T cells by flow cytometry. Mean and standard error of the mean are shown. feeble, Slc15a4feeble/feeble mice; inept, IRF7inept/inept mice. Representative data of 2 independent experiments are shown. n≥4 per group of mice. Unless marked, p>0.05 between WT and feeble and not statistically significantly different.
Figure 3. Defect in antigen specific feeble T cell responses is extrinsic.
Equal numbers of bone marrow progenitors were harvested from WT and feeble mice and transferred into feeble recipients to generate mixed bone marrow chimeras. >8 weeks post irradiation, mixed bone marrow chimeras were challenged with LCMV Cl13. Eight days post infection splenocytes were harvested, counted, cultured with the indicated peptides for 5 hours, stained with antibodies, and analyzed by flow cytometry IFN-γ production in CD8+ (A) and CD4+ (B) antigen specific T cells. In (C) mice were primed with TAP1−/− 5E1 fibroblasts expressing the model antigen from Adeno E1B. Seven days after i.p. injection splenocytes from mixed bone marrow chimeras (prepared as above) were cultured ex vivo with the immunodominant antigen for 5 hours. Then IFN-γ expression was quantitated in CD8+ T cells by flow cytometry. Mean and standard error of the mean are shown. Feeble, Slc15a4feeble/feeble mice. n = 5 per group of mice. 1 of 2 similar experiments is shown. Unless marked, p>0.05 between WT and feeble and not statistically significantly different.
Figure 4. Slc15a4 is required for specific acute inflammatory cytokine production.
One and 8 days post LCMV Cl13 infection serum was collected and the cytokines displayed were quantitated from WT and feeble mice. Individual replicates, mean and standard error of the mean are shown. Representative data of 2 independent experiments are shown; n = 5 per group of infected mice. Unless marked, p>0.05 between WT and feeble and not statistically significantly different.
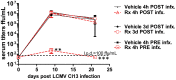
Figure 5. Prophylactic treatment with CpG-DOTAP prevents persistent viral infection.
WT mice were treated with vehicle or i.v. 2 µg CpG-ODN in DOTAP (“Rx”) either 4 h prior, 4 h post or 3 days post infection with LCMV Cl13. Serum was collected at the indicated times post infection and titered for infectious virus. CpG-DOTAP: CpG-A plus DOTAP (Roche); l.o.d., limit of detection. Mean and standard error of the mean are shown for 5 mice per group of infected mice. 1 of 2 similar experiments is shown. Unless marked, p>0.05 between treatment groups and not statistically significantly different.
Similar articles
- Plasmacytoid dendritic cells control T-cell response to chronic viral infection.
Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. Cervantes-Barragan L, et al. Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):3012-7. doi: 10.1073/pnas.1117359109. Epub 2012 Feb 6. Proc Natl Acad Sci U S A. 2012. PMID: 22315415 Free PMC article. - Interleukin-27R Signaling Mediates Early Viral Containment and Impacts Innate and Adaptive Immunity after Chronic Lymphocytic Choriomeningitis Virus Infection.
Harker JA, Wong KA, Dallari S, Bao P, Dolgoter A, Jo Y, Wehrens EJ, Macal M, Zuniga EI. Harker JA, et al. J Virol. 2018 May 29;92(12):e02196-17. doi: 10.1128/JVI.02196-17. Print 2018 Jun 15. J Virol. 2018. PMID: 29593047 Free PMC article. - Conventional but not plasmacytoid dendritic cells foster the systemic virus-induced type I IFN response needed for efficient CD8 T cell priming.
Hervas-Stubbs S, Riezu-Boj JI, Mancheño U, Rueda P, Lopez L, Alignani D, Rodríguez-García E, Thieblemont N, Leclerc C. Hervas-Stubbs S, et al. J Immunol. 2014 Aug 1;193(3):1151-61. doi: 10.4049/jimmunol.1301440. Epub 2014 Jun 27. J Immunol. 2014. PMID: 24973449 Free PMC article. - Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance.
Swiecki M, Colonna M. Swiecki M, et al. Immunol Rev. 2010 Mar;234(1):142-62. doi: 10.1111/j.0105-2896.2009.00881.x. Immunol Rev. 2010. PMID: 20193017 Free PMC article. Review. - The role of dendritic cells in viral persistence.
Ng CT, Sullivan BM, Oldstone MB. Ng CT, et al. Curr Opin Virol. 2011 Sep;1(3):160-6. doi: 10.1016/j.coviro.2011.05.006. Curr Opin Virol. 2011. PMID: 21909344 Free PMC article. Review.
Cited by
- A requirement for slc15a4 in imiquimod-induced systemic inflammation and psoriasiform inflammation in mice.
Griffith AD, Zaidi AK, Pietro A, Hadiono M, Yang JS, Davis R, Popkin DL. Griffith AD, et al. Sci Rep. 2018 Sep 27;8(1):14451. doi: 10.1038/s41598-018-32668-9. Sci Rep. 2018. PMID: 30262916 Free PMC article. - Slc15a4 function is required for intact class switch recombination to IgG2c in response to TLR9 stimulation.
Dosenovic P, Ádori M, Adams WC, Pedersen GK, Soldemo M, Beutler B, Karlsson Hedestam GB. Dosenovic P, et al. Immunol Cell Biol. 2015 Feb;93(2):136-46. doi: 10.1038/icb.2014.82. Epub 2014 Oct 14. Immunol Cell Biol. 2015. PMID: 25310967 - Induction of Systemic Autoimmunity by a Xenobiotic Requires Endosomal TLR Trafficking and Signaling from the Late Endosome and Endolysosome but Not Type I IFN.
Pollard KM, Escalante GM, Huang H, Haraldsson KM, Hultman P, Christy JM, Pawar RD, Mayeux JM, Gonzalez-Quintial R, Baccala R, Beutler B, Theofilopoulos AN, Kono DH. Pollard KM, et al. J Immunol. 2017 Dec 1;199(11):3739-3747. doi: 10.4049/jimmunol.1700332. Epub 2017 Oct 20. J Immunol. 2017. PMID: 29055005 Free PMC article. - Natural Killer Cell Sensing of Infected Cells Compensates for MyD88 Deficiency but Not IFN-I Activity in Resistance to Mouse Cytomegalovirus.
Cocita C, Guiton R, Bessou G, Chasson L, Boyron M, Crozat K, Dalod M. Cocita C, et al. PLoS Pathog. 2015 May 8;11(5):e1004897. doi: 10.1371/journal.ppat.1004897. eCollection 2015 May. PLoS Pathog. 2015. PMID: 25954804 Free PMC article. - Metabolic control from the endolysosome: lysosome-resident amino acid transporters open novel therapeutic possibilities.
Kobayashi T, Toyama-Sorimachi N. Kobayashi T, et al. Front Immunol. 2023 Sep 15;14:1243104. doi: 10.3389/fimmu.2023.1243104. eCollection 2023. Front Immunol. 2023. PMID: 37781390 Free PMC article. Review.
References
- Colonna M, Krug A, Cella M (2002) Interferon-producing cells: on the front line in immune responses against pathogens. Curr Opin Immunol 14: 373–379. - PubMed
- Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, et al. (2001) Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol 2: 1144–1150. - PubMed
- Bjorck P (2001) Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98: 3520–3526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases