Engagement of Siglec-7 receptor induces a pro-inflammatory response selectively in monocytes - PubMed (original) (raw)
Engagement of Siglec-7 receptor induces a pro-inflammatory response selectively in monocytes
Stefania Varchetta et al. PLoS One. 2012.
Abstract
Sialic acid binding immunoglobulin-like lectin-7 (Siglec-7) is a trans-membrane receptor carrying immunoreceptor tyrosine based inhibitory motifs (ITIMs) and delivering inhibitory signals upon ligation with sialylated glycans. This inhibitory function can be also targeted by several pathogens that have evolved to express sialic acids on their surface to escape host immune responses. Here, we demonstrate that cross-linking of Siglec-7 by a specific monoclonal antibody (mAb) induces a remarkably high production of IL-6, IL-1α, CCL4/MIP-1β, IL-8 and TNF-α. Among the three immune cell subsets known to constitutively express Siglec-7, the production of these pro-inflammatory cytokines and chemokines selectively occurs in monocytes and not in Natural Killer or T lymphocytes. This Siglec-7-mediated activating function is associated with the phosphorylation of the extracellular signal-regulated kinase (ERK) pathway. The present study also shows that sialic acid-free Zymosan yeast particles are able to bind Siglec-7 on monocytes and that this interaction mimics the ability of the anti Siglec-7 mAb to induce the production of pro-inflammatory mediators. Indeed, blocking or silencing Siglec-7 in primary monocytes greatly reduced the production of inflammatory cytokines and chemokines in response to Zymosan, thus confirming that Siglec-7 participates in generating a monocyte-mediated inflammatory outcome following pathogen recognition. The presence of an activating form of Siglec-7 in monocytes provides the host with a new and alternative mechanism to encounter pathogens not expressing sialylated glycans.
Conflict of interest statement
Competing Interests: The authors declare no competing financial interests.
Figures
Figure 1. Detection of cytokines in PBMC supernatant upon engagement of Siglec-7.
Secretion of IL-6, IL-1α, IL-8, CCL4/MIP-1β, GRO and DAN in the supernatant of PBMCs stimulated with the anti-Siglec-7 mAb (right panel) compared with that of PBMCs incubated with a matched IgG2b isotype control (left panel). The culture medium was collected and analysed using a semi-quantitative protein array detecting simultaneously 507 human soluble proteins. Data shown in this figure are representative of 3 independent experiments.
Figure 2. Intracellular production of pro-inflammatory cytokines and chemokines in monocytes upon engagement of Siglec-7.
(A) Representative flow cytometry dot plot graphs showing the percentage of CD14pos monocytes, within total PBMCs, producing IL-6, IL-1α, CCL4/MIP-1β, IL-8 and TNF-α after incubation with either the anti-Siglec-7 mAb (lower line) or the matched IgG2b isotype control (upper line). (B) Statistical summary graphs of dot plots with medians (horizontal black bars) and p values showing the percentage of CD14pos monocytes producing IL-6, IL-1α, MIP-1β, IL-8 and TNF-α in response to either the anti-Siglec-7 mAb (red circles) or the matched IgG2b isotype control (green circles).
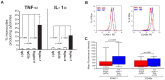
Figure 3. Intracellular production of TNF-α and IL-1α in monocytes upon engagement of Siglec-7 and Siglec-9 and modulation of adhesion molecules in monocytes upon engagement of Siglec-7.
(A) Statistical histogram bar graph showing the percentages of CD14pos monocyte producing TNF-α (left) and IL-1α (right) in the presence of mAbs cross-linking either Siglec-7 and Siglec-9 or their relative IgG2b and IgG1 isotype controls. Data are representative of 5 independent experiments performed in triplicates (± SD). (B) Representative flow cytometry histogram graphs showing the mean fluorescence intensity (MFI) of ICAM-1 (left) and CD49e (right) on CD14pos monocyte after incubation with either the anti-Siglec-7 mAb (blue lines) or with the matched IgG2b isotype control (red lines). (C) Statistical summary graphs of box plots with medians and standard deviation showing the MFI of ICAM-1 (left) and CD49e (right) on CD14pos monocyte after incubation with either the anti-Siglec-7 mAb (blue boxes) or with the matched IgG2b isotype control (red boxes). Data are representative of 5 independent experiments (± SD).
Figure 4. Phosphorylation of ERK upon engagement of Siglec-7 in monocytes.
(A) Representative phospho-flow cytometry dot plot graphs showing the percentage of phosphorylation of p38 MAPK and of ERK 1–2 in freshly purified monocytes incubated with an IgG2b isotype (left column) and anti Siglec-7 mAb (right column) at time 0 (upper line) and after 5 (middle line) and 15 (lower line) minutes of incubation. The number highlighted in bold red within the lower right quadrant of the dot plot graph located in the lower line of right column indicates the phosphorylation of ERK following ligation of Siglec-7. (B) Representative western blot image showing the phoshorylation of ERK 1–2 in freshly purified monocytes stimulated with IgG2b isotype (left) and anti Siglec-7 mAb (right) after 15 minutes of incubation. Data are representative of 3 independent experiments (± SD).
Figure 5. Binding of pathogens to Siglec-7 Fc chimera.
Representative flow cytometric dot plot graphs showing the binding of goat anti human (GAH) Fc Ab (upper line) and of Siglec-7 Fc chimera (lower line) to Escherichia coli (strains K1 and K12) and Candida albicans. Data are representative of 3 independent experiments.
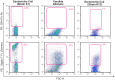
Figure 6. Binding of Zymosan to Siglec-7 Fc chimera and to Siglec-7 receptor expressed on freshly putified monocytes.
(A) Representative flow cytometric dot plot graphs showing the binding of goat anti human (GAH) Fc Ab (left), NKp44 Fc chimera (middle) and Siglec-7 Fc chimera (right) to Zymosan. (B) Fluorescent microscopic images of Zymosan particles (gray, left part of the panel) surrounded by PE-labeled Siglec-7 Fc chimera (green, right part of the panel). (C) Fluorescent microscopic images of primary monocytes labeled for Siglec-7 (red) and incubated with Zymosan particles (green) for 5 and 30 minutes. The co-localization is labeled in yellow.
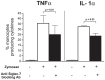
Figure 7. _Zymosan_-induced production of TNFα and IL-1α:masking of Siglec-7.
Statistical histogram bar graph showing the percentages of CD14pos monocyte producing TNF-α (left) and IL-1α (right) either in the absence (gray bars) or in the presence (white bars) of Zymosan and in presence of Zymosan cultured with blocking anti-Siglec-7 Abs (black bars). The intracellular production of TNF-α and IL-1α were evaluated by flow cytometric analysis. Data are representative of 5 independent experiments performed in triplicates (± SD).
Figure 8. Zymosan-induced production of TNFα and IL-1α: silencing of Siglec-7.
(A) Representative graphs showing the intracellular production of TNF-α and IL-1α in monocytes transfected either with non-targeting SiRNA control probes (green line in the histogram graph and dot plot graphs in the upper line of the panel) or with SiRNA duplexes specific for Siglec-7 (red line in the histogram graph and dot plot graphs in the lower line of the panel) in response to Zymosan. MFI: Mean Fluoresence Intensity. Filled gray Histogram: isotype control. (B) Summary graphs of dot plots (left) and statistical summary bar graphs with p values and standard deviation (right) showing the percentages of decrement of TNF-α (left) and IL-1α (right) in monocytes silenced for Siglec-7 compared to monocytes not silenced for Siglec-7 in response to Zymosan. Data are representative of 5 independent experiments (± SD).
Similar articles
- Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9.
Ikehara Y, Ikehara SK, Paulson JC. Ikehara Y, et al. J Biol Chem. 2004 Oct 8;279(41):43117-25. doi: 10.1074/jbc.M403538200. Epub 2004 Aug 3. J Biol Chem. 2004. PMID: 15292262 - Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus.
Ali SR, Fong JJ, Carlin AF, Busch TD, Linden R, Angata T, Areschoug T, Parast M, Varki N, Murray J, Nizet V, Varki A. Ali SR, et al. J Exp Med. 2014 Jun 2;211(6):1231-42. doi: 10.1084/jem.20131853. Epub 2014 May 5. J Exp Med. 2014. PMID: 24799499 Free PMC article. - Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation?
Cao H, Crocker PR. Cao H, et al. Immunology. 2011 Jan;132(1):18-26. doi: 10.1111/j.1365-2567.2010.03368.x. Epub 2010 Nov 11. Immunology. 2011. PMID: 21070233 Free PMC article. Review. - Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors.
Bochner BS. Bochner BS. Clin Exp Allergy. 2009 Mar;39(3):317-24. doi: 10.1111/j.1365-2222.2008.03173.x. Clin Exp Allergy. 2009. PMID: 19178537 Free PMC article. Review.
Cited by
- Plasma Glycomic Markers of Accelerated Biological Aging During Chronic HIV Infection.
Giron LB, Liu Q, Adeniji OS, Yin X, Kannan T, Ding J, Lu DY, Langan S, Zhang J, Azevedo JLLC, Li SH, Shalygin S, Azadi P, Hanna DB, Ofotokun I, Lazar J, Fischl MA, Haberlen S, Macatangay B, Adimora AA, Jamieson BD, Rinaldo C, Merenstein D, Roan NR, Kutsch O, Gange S, Wolinsky S, Witt M, Post WS, Kossenkov A, Landay A, Frank I, Tien PC, Gross R, Brown TT, Abdel-Mohsen M. Giron LB, et al. bioRxiv [Preprint]. 2023 Dec 31:2023.08.09.551369. doi: 10.1101/2023.08.09.551369. bioRxiv. 2023. PMID: 37609144 Free PMC article. Updated. Preprint. - Neuraminidase inhibition improves endothelial function in diabetic mice.
Foote CA, Ramirez-Perez FI, Smith JA, Ghiarone T, Morales-Quinones M, McMillan NJ, Augenreich MA, Power G, Burr K, Aroor AR, Bender SB, Manrique-Acevedo C, Padilla J, Martinez-Lemus LA. Foote CA, et al. Am J Physiol Heart Circ Physiol. 2023 Dec 1;325(6):H1337-H1353. doi: 10.1152/ajpheart.00337.2023. Epub 2023 Oct 6. Am J Physiol Heart Circ Physiol. 2023. PMID: 37801046 Free PMC article. - Targeted delivery of mycobacterial antigens to human dendritic cells via Siglec-7 induces robust T cell activation.
Kawasaki N, Rillahan CD, Cheng TY, Van Rhijn I, Macauley MS, Moody DB, Paulson JC. Kawasaki N, et al. J Immunol. 2014 Aug 15;193(4):1560-6. doi: 10.4049/jimmunol.1303278. Epub 2014 Jul 7. J Immunol. 2014. PMID: 25000981 Free PMC article. - Siglec-7 on peripheral blood eosinophils: Surface expression and function.
Legrand F, Landolina N, Zaffran I, Emeh RO, Chen E, Klion AD, Levi-Schaffer F. Legrand F, et al. Allergy. 2019 Jul;74(7):1257-1265. doi: 10.1111/all.13730. Epub 2019 Feb 26. Allergy. 2019. PMID: 30690753 Free PMC article. - Blockade of novel immune checkpoints and new therapeutic combinations to boost antitumor immunity.
Archilla-Ortega A, Domuro C, Martin-Liberal J, Muñoz P. Archilla-Ortega A, et al. J Exp Clin Cancer Res. 2022 Feb 14;41(1):62. doi: 10.1186/s13046-022-02264-x. J Exp Clin Cancer Res. 2022. PMID: 35164813 Free PMC article. Review.
References
- Crocker PR, Paulson JC, Varki A (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7: 255–266. - PubMed
- Jacobs T, Erdmann H, Fleischer B (2010) Molecular interaction of Siglecs (sialic acid-binding Ig-like lectins) with sialylated ligands on Trypanosoma cruzi. Eur J Cell Biol 89: 113–116. - PubMed
- Jones C, Virji M, Crocker PR (2003) Recognition of sialylated meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads to enhanced bacterial uptake. Mol Microbiol 49: 1213–1225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by the Intramural Research program of Istituto Clinico Humanitas (Grant 20090917 to D.M.), and by the Italian Ministry of Health (Ricerca Finalizzata, Bando ISS, Grants RF-ICH-2009-1299677 to D.M. and RF-ICH-2009-1304134 and to J.M). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous