The CTX-M-15-producing Escherichia coli clone O25b: H4-ST131 has high intestine colonization and urinary tract infection abilities - PubMed (original) (raw)
The CTX-M-15-producing Escherichia coli clone O25b: H4-ST131 has high intestine colonization and urinary tract infection abilities
Sophie Vimont et al. PLoS One. 2012.
Abstract
Increasing numbers of pyelonephritis-associated uropathogenic Escherichia coli (UPEC) are exhibiting high resistance to antibiotic therapy. They include a particular clonal group, the CTX-M-15-producing O25b:H4-ST131 clone, which has been shown to have a high dissemination potential. Here we show that a representative isolate of this E. coli clone, referred to as TN03, has enhanced metabolic capacities, acts as a potent intestine- colonizing strain, and displays the typical features of UPEC strains. In a modified streptomycin-treated mouse model of intestinal colonization where streptomycin was stopped 5 days before inoculation, we show that TN03 outcompetes the commensal E. coli strains K-12 MG1655, IAI1, and ED1a at days 1 and 7. Using an experimental model of ascending UTI in C3H/HeN mice, we then show that TN03 colonized the urinary tract. One week after the transurethral inoculation of the TN03 isolates, the bacterial loads in the bladder and kidneys were significantly greater than those of two other UPEC strains (CFT073 and HT7) belonging to the same B2 phylogenetic group. The differences in bacterial loads did not seem to be directly linked to differences in the inflammatory response, since the intrarenal expression of chemokines and cytokines and the number of polymorphonuclear neutrophils attracted to the site of inflammation was the same in kidneys colonized by TN03, CFT073, or HT7. Lastly, we show that in vitro TN03 has a high maximum growth rate in both complex (Luria-Bertani and human urine) and minimum media. In conclusion, our findings indicate that TN03 is a potent UPEC strain that colonizes the intestinal tract and may persist in the kidneys of infected hosts.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
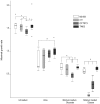
Figure 1. Intestinal colonization.
Intestinal competition tests were performed in streptomycin-pre-treated CD1 mice. Competitive indexes (CIs) are given for day 1 and day 7 post-inoculation. The CI index is a ratio of ratios, in which the ratio between resistant (TN03) and sensitive (K-12 MG1655, IAI1 or ED1a) strains at post-inoculation time points is divided by this same ratio at the initial inoculation time. Horizontal bars represent median log10 CI ratios and compared to a ratio of null effect (0, that is log10 1.0) using a Wilcoxon signed-rank test.
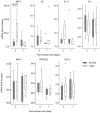
Figure 2. Maximum growth rate in four media.
Four E. coli strains: K-12 MG1655 (white), HT7 (light grey), CFT073 (grey) and TN03 (dark grey) were grown in four different media: Luria Bertani (LB), urine, minimum medium with gluconate and minimum medium with glucose. Boxplots represent distribution of maximal growth rates (MGRs) calculated during the three repetitions of culture assays with the smooth spline function from R network. Black bars inside each boxplot represent medians. Dots located far from the box represent outliers. Links between boxplots with asterisks represent significant differences between two strains tested by Welch test for observed mean comparison. * p<0.05.
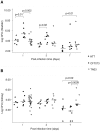
Figure 3. Experimental model of UTI.
C3H/HeN mice were infected with the HT7, CFT073, or TN03 strains directly by transurethral inoculation of the bacteria (108 cfu in 50 µl sterile PBS). At day 1, day 2 and day 7 post infection, bladders (A) and kidneys (B) were harvested and their bacterial burdens determined. Horizontal bars represent median log10 bacterial counts. Statistical differences between strains at each post-infection time point were performed using the Kruskal-Wallis equality-of-populations rank test.
Figure 4. Expression of pro-inflammatory mediators in kidneys infected by UPEC.
Expression of MIP-2, KC, IL-1ß, IL-6, MCP-1, RANTES and TNF-α in the day 2 and day 7 post-infection kidneys following the transurethral inoculation of the CFT073 and TN03 UPEC isolates. mRNA expression of each inflammatory marker was calculated using the −ΔΔCT method. Values are expressed as the relative fold increase in kidneys of each mRNA level of proinflammatory mediator in comparison with that measured in naive mice. Horizontal bars represent the median values. Statistical differences between the strains were determined at each time point using the Kruskal-Wallis equality-of-populations rank test.
Similar articles
- Kinetics of uropathogenic Escherichia coli metapopulation movement during urinary tract infection.
Walters MS, Lane MC, Vigil PD, Smith SN, Walk ST, Mobley HL. Walters MS, et al. mBio. 2012 Feb 7;3(1):e00303-11. doi: 10.1128/mBio.00303-11. Print 2012. mBio. 2012. PMID: 22318320 Free PMC article. - Characterization of O25b-ST131 Escherichia coli Clone Producing CTX-M-15, DHA-4, and CMY-42 in Urinary Tract Infections in a Tunisian Island.
Dziri O, Dziri R, Maraoub A, Chouchani C. Dziri O, et al. Microb Drug Resist. 2020 Jul;26(7):741-746. doi: 10.1089/mdr.2019.0076. Epub 2020 Jan 9. Microb Drug Resist. 2020. PMID: 31916915 - Recent emergence of clonal group O25b:K1:H4-B2-ST131 ibeA strains among Escherichia coli poultry isolates, including CTX-M-9-producing strains, and comparison with clinical human isolates.
Mora A, Herrera A, Mamani R, López C, Alonso MP, Blanco JE, Blanco M, Dahbi G, García-Garrote F, Pita JM, Coira A, Bernárdez MI, Blanco J. Mora A, et al. Appl Environ Microbiol. 2010 Nov;76(21):6991-7. doi: 10.1128/AEM.01112-10. Epub 2010 Sep 3. Appl Environ Microbiol. 2010. PMID: 20817805 Free PMC article. - Metabolic Adaptations of Uropathogenic E. coli in the Urinary Tract.
Mann R, Mediati DG, Duggin IG, Harry EJ, Bottomley AL. Mann R, et al. Front Cell Infect Microbiol. 2017 Jun 8;7:241. doi: 10.3389/fcimb.2017.00241. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28642845 Free PMC article. Review. - Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4.
Peirano G, Pitout JD. Peirano G, et al. Int J Antimicrob Agents. 2010 Apr;35(4):316-21. doi: 10.1016/j.ijantimicag.2009.11.003. Epub 2010 Jan 13. Int J Antimicrob Agents. 2010. PMID: 20060273 Review.
Cited by
- Epidemiology and characteristics of Escherichia coli sequence type 131 (ST131) from long-term care facility residents colonized intestinally with fluoroquinolone-resistant Escherichia coli.
Han JH, Garrigan C, Johnston B, Nachamkin I, Clabots C, Bilker WB, Santana E, Tolomeo P, Maslow J, Myers J, Carson L, Lautenbach E, Johnson JR; CDC Prevention Epicenters Program. Han JH, et al. Diagn Microbiol Infect Dis. 2017 Mar;87(3):275-280. doi: 10.1016/j.diagmicrobio.2016.11.016. Epub 2016 Nov 28. Diagn Microbiol Infect Dis. 2017. PMID: 27939288 Free PMC article. - Structural Alteration of OmpR as a Source of Ertapenem Resistance in a CTX-M-15-Producing Escherichia coli O25b:H4 Sequence Type 131 Clinical Isolate.
Dupont H, Choinier P, Roche D, Adiba S, Sookdeb M, Branger C, Denamur E, Mammeri H. Dupont H, et al. Antimicrob Agents Chemother. 2017 Apr 24;61(5):e00014-17. doi: 10.1128/AAC.00014-17. Print 2017 May. Antimicrob Agents Chemother. 2017. PMID: 28264855 Free PMC article. - Intensity and Mechanisms of Fluoroquinolone Resistance within the H30 and H30Rx Subclones of Escherichia coli Sequence Type 131 Compared with Other Fluoroquinolone-Resistant E. coli.
Johnson JR, Johnston B, Kuskowski MA, Sokurenko EV, Tchesnokova V. Johnson JR, et al. Antimicrob Agents Chemother. 2015 Aug;59(8):4471-80. doi: 10.1128/AAC.00673-15. Epub 2015 May 18. Antimicrob Agents Chemother. 2015. PMID: 25987621 Free PMC article. - The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017.
Critchley IA, Cotroneo N, Pucci MJ, Mendes R. Critchley IA, et al. PLoS One. 2019 Dec 10;14(12):e0220265. doi: 10.1371/journal.pone.0220265. eCollection 2019. PLoS One. 2019. PMID: 31821338 Free PMC article. - Repeated Isolation of Extended-Spectrum-β-Lactamase-Positive Escherichia coli Sequence Types 648 and 131 from Community Wastewater Indicates that Sewage Systems Are Important Sources of Emerging Clones of Antibiotic-Resistant Bacteria.
Paulshus E, Thorell K, Guzman-Otazo J, Joffre E, Colque P, Kühn I, Möllby R, Sørum H, Sjöling Å. Paulshus E, et al. Antimicrob Agents Chemother. 2019 Aug 23;63(9):e00823-19. doi: 10.1128/AAC.00823-19. Print 2019 Sep. Antimicrob Agents Chemother. 2019. PMID: 31235629 Free PMC article.
References
- Gupta K, Scholes D, Stamm WE (1999) Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. Jama 281: 736–738. - PubMed
- Pitout JD, Nordmann P, Laupland KB, Poirel L (2005) Emergence of Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) in the community. J Antimicrob Chemother 56: 52–59. - PubMed
- Rodriguez-Bano J, Paterson DL (2006) A change in the epidemiology of infections due to extended-spectrum beta-lactamase-producing organisms. Clin Infect Dis 42: 935–937. - PubMed
- Pitout JD, Laupland KB (2008) Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8: 159–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was funded in part by a grant from the French National Research Agency under reference ANR-08-MIE-030 (to GA, and AV). AV was in receipt of a “Contrat Hospitalier de Recherche Translationnelle” (CHRP, INSERM-APHP, 2010). A.B. was supported by a “Bourse Médico-Scientifique” from the “Fondation pour la Recherche Médicale”. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical