Cytosolic [Ca2+] regulation of InsP3-evoked puffs - PubMed (original) (raw)
Cytosolic [Ca2+] regulation of InsP3-evoked puffs
Michiko Yamasaki-Mann et al. Biochem J. 2013.
Abstract
InsP3-mediated puffs are fundamental building blocks of cellular Ca2+ signalling, and arise through the concerted opening of clustered InsP3Rs (InsP3 receptors) co-ordinated via Ca2+-induced Ca2+ release. Although the Ca2+ dependency of InsP3Rs has been extensively studied at the single channel level, little is known as to how changes in basal cytosolic [Ca2+] would alter the dynamics of InsP3-evoked Ca2+ signals in intact cells. To explore this question, we expressed Ca2+-permeable channels (nicotinic acetylcholine receptors) in the plasma membrane of voltage-clamped Xenopus oocytes to regulate cytosolic [Ca2+] by changing the electrochemical gradient for extracellular Ca2+ entry, and imaged Ca2+ liberation evoked by photolysis of caged InsP3. Elevation of basal cytosolic [Ca2+] strongly increased the amplitude and shortened the latency of global Ca2+ waves. In oocytes loaded with EGTA to localize Ca2+ signals, the number of sites at which puffs were observed and the frequency and latency of puffs were strongly dependent on cytosolic [Ca2+], whereas puff amplitudes were only weakly affected. The results of the present study indicate that basal cytosolic [Ca2+] strongly affects the triggering of puffs, but has less of an effect on puffs once they have been initiated.
Figures
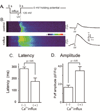
FIGURE 1. Elevated basal cytosolic [Ca2+] enhances InsP3-induced Ca2+ waves
(A) Schematic of the experimental protocol. (B) Representative confocal linescan images illustrating fluo-4 dextran fluorescence signals evoked by photoreleased InsP3 under control conditions without elevation of cytosolic [Ca2+] (upper) and with cytosolic [Ca2+] elevation (lower). Increasing fluorescence (ΔF/Fo: Ca2+ level) is depicted on a pseudocolor scale as indicated by the colour bar. Traces on the right show corresponding fluorescence profiles averaged across 15 µm widths of the linescans (indicated by bars). (C) Mean values of latency between the photolysis flash and initial rise in fluorescence derived from traces like those in (B). Latency without Ca2+ influx 284 ± 14 ms; during Ca2+ influx 177 ± 30 ms, p < 0.05. (D) Mean peak amplitudes of Ca2+ waves derived from traces like those in (B). ΔF/Fo without Ca2+ influx 1.68 ± 0.23; during Ca2+ influx 3.50 ± 0.45, p < 0.05: n = 6 and 4 oocytes, respectively).
FIGURE 2. Cytosolic [Ca2+]-dependent potentiation of InsP3-evoked Ca2+ puffs
(A) (a) schematic of the experimental protocol. (b,c) Representative fluorescence profiles of puffs evoked, respectively, without (control) and with (+ influx) basal cytosolic [Ca2+] elevation, obtained from the same oocyte. The record in (b) was obtained from the single responding site within the image field. That in (c) shows superimposed traces from 7 responding sites. (d) Zoomed version of (c) on an expanded timescale to illustrate more clearly the variation in puff latencies following photorelease of InsP3. Traces in (b–d) are blanked out during the photolysis flash. (B) Scatter plot showing the numbers of sites within the imaging field that showed puffs following weak (open symbols; 25–50 ms flash duration) or strong (filled symbols; 50–100 ms) photorelease of InsP3 as a function of cytosolic Ca2+ elevation during influx (ΔF/Fo[Ca2+]cyt). (C) Mean numbers of responding puff sites within imaging field, grouped by photolysis strength (weak, open bars; strong, filed bars) and by elevation of basal cytosolic [Ca2+] (ΔF/Fo < 0.1 or > 0.1).
FIGURE 3. Puff latencies shorten with increasing cytosolic [Ca2+]
Latencies were measured as the time from end of the photolysis flash to the observation of the first puff at a given site. (A, B) Mean latencies of puffs evoked, respectively, by weak and strong photorelease of InsP3. Open circles in (A) and filled squares in (B) indicate data from individual puffs; bars indicate mean ± SEM. (C, D) Histograms showing distributions of latencies of puffs evoked by strong stimuli during cytosolic Ca2+ elevations < 0.1 ΔF/F0 (**C**) and > 0.1 (D) Curves are single exponential fits to the data with respective time constants of 1414 ± 391 ms and 575 ± 93 ms.
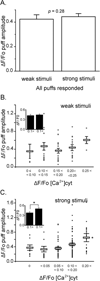
FIGURE 4. The amplitude of InsP3-evoked puffs is only weakly dependent on the basal cytosolic [Ca2+]
(A) Mean puff amplitudes evoked by weak photorelease of InsP3 (ΔF/Fo = 0.42 ± 0.04, n = 56) and strong photorelease (ΔF/Fo = 0.44 ± 0.23, n = 146. p > 0.05), after pooling data across all basal cytosolic [Ca2+] levels. (B) Main panel shows a scatter plot of amplitudes (ΔF/F0) of puffs evoked by weak photorelease of InsP3 as a function of increase in basal fluorescence during Ca2+ influx. Open circles mark data from individual puffs and bars show mean ± SEM. (C) Corresponding measurements of puff amplitudes following strong photorelease of InsP3. Inset graphs in (B) and (C) represent mean values of puff amplitudes evoked, respectively, by weak and strong photolysis flashes grouped for cytosolic [Ca2+] elevations < 0.1 and > 0.1 ΔF/Fo.
FIGURE 5. Duration of InsP3-evoked puffs is independent on the basal cytosolic [Ca2+]
(A) Scatter plot showing full-duration at half-maximal amplitude (FDHM) of all puffs observed within the imaging field as a function of their latencies. Open circles are control puffs evoked by the strong photolysis flash, and filled circles represents FDHM of puffs observed during Ca2+ influx (mean ΔF/Fo[Ca2+]cyt = 0.23 ± 0.03, 4 trials). (B) Mean FDHM of puffs. (control FDHM; 64.8 ± 5.2, n = 25, with influx; FDHM 64.8 ± 5.2, n = 44, 4 oocytes).
Similar articles
- Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes.
Yao Y, Choi J, Parker I. Yao Y, et al. J Physiol. 1995 Feb 1;482 ( Pt 3)(Pt 3):533-53. doi: 10.1113/jphysiol.1995.sp020538. J Physiol. 1995. PMID: 7738847 Free PMC article. - Activation and co-ordination of InsP3-mediated elementary Ca2+ events during global Ca2+ signals in Xenopus oocytes.
Callamaras N, Marchant JS, Sun XP, Parker I. Callamaras N, et al. J Physiol. 1998 May 15;509 ( Pt 1)(Pt 1):81-91. doi: 10.1111/j.1469-7793.1998.081bo.x. J Physiol. 1998. PMID: 9547383 Free PMC article. - Fast kinetics of calcium liberation induced in Xenopus oocytes by photoreleased inositol trisphosphate.
Parker I, Yao Y, Ilyin V. Parker I, et al. Biophys J. 1996 Jan;70(1):222-37. doi: 10.1016/S0006-3495(96)79565-6. Biophys J. 1996. PMID: 8770200 Free PMC article. - Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips.
Parker I, Choi J, Yao Y. Parker I, et al. Cell Calcium. 1996 Aug;20(2):105-21. doi: 10.1016/s0143-4160(96)90100-1. Cell Calcium. 1996. PMID: 8889202 Review. - Calcium puffs in Xenopus oocytes.
Parker I, Yao Y. Parker I, et al. Ciba Found Symp. 1995;188:50-60; discussion 60-5. doi: 10.1002/9780470514696.ch4. Ciba Found Symp. 1995. PMID: 7587623 Review.
Cited by
- IP3 mediated global Ca2+ signals arise through two temporally and spatially distinct modes of Ca2+ release.
Lock JT, Parker I. Lock JT, et al. Elife. 2020 May 12;9:e55008. doi: 10.7554/eLife.55008. Elife. 2020. PMID: 32396066 Free PMC article. - Amyloid-beta Alzheimer targets - protein processing, lipid rafts, and amyloid-beta pores.
Arbor SC, LaFontaine M, Cumbay M. Arbor SC, et al. Yale J Biol Med. 2016 Mar 24;89(1):5-21. eCollection 2016 Mar. Yale J Biol Med. 2016. PMID: 27505013 Free PMC article. Review. - Intracellular calcium channels: inositol-1,4,5-trisphosphate receptors.
Fedorenko OA, Popugaeva E, Enomoto M, Stathopulos PB, Ikura M, Bezprozvanny I. Fedorenko OA, et al. Eur J Pharmacol. 2014 Sep 15;739:39-48. doi: 10.1016/j.ejphar.2013.10.074. Epub 2013 Dec 1. Eur J Pharmacol. 2014. PMID: 24300389 Free PMC article. Review. - Obstruction of ventricular Ca2+ -dependent arrhythmogenicity by inositol 1,4,5-trisphosphate-triggered sarcoplasmic reticulum Ca2+ release.
Blanch I Salvador J, Egger M. Blanch I Salvador J, et al. J Physiol. 2018 Sep;596(18):4323-4340. doi: 10.1113/JP276319. Epub 2018 Aug 7. J Physiol. 2018. PMID: 30004117 Free PMC article. - Frequency and relative prevalence of calcium blips and puffs in a model of small IP₃R clusters.
Qi H, Huang Y, Rüdiger S, Shuai J. Qi H, et al. Biophys J. 2014 Jun 3;106(11):2353-63. doi: 10.1016/j.bpj.2014.04.027. Biophys J. 2014. PMID: 24896114 Free PMC article.
References
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
- Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. - PubMed
- DeLisle S, Welsh MJ. Inositol trisphosphate is required for the propagation of calcium waves in Xenopus oocytes. J. Biol. Chem. 1992;267:7963–7966. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous