Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal - PubMed (original) (raw)
Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal
Graziano Martello et al. Cell Stem Cell. 2012.
Erratum in
- Cell Stem Cell. 2013 May 2;12(5):630
Abstract
Inhibition of glycogen synthase kinase-3 (Gsk3) supports mouse embryonic stem cells (ESCs) by modulating Tcf3, but the critical targets downstream of Tcf3 are unclear. We analyzed the intersection between genome localization and transcriptome data sets to identify genes repressed by Tcf3. Among these, manipulations of Esrrb gave distinctive phenotypes in functional assays. Knockdown and knockout eliminated response to Gsk3 inhibition, causing extinction of pluripotency markers and loss of colony forming capability. Conversely, forced expression phenocopied Gsk3 inhibition or Tcf3 deletion by suppressing differentiation and sustaining self-renewal. Thus the nuclear receptor Esrrb is necessary and sufficient to mediate self-renewal downstream of Gsk3 inhibition. Leukaemia inhibitory factor (LIF) regulates ESCs through Stat3, independently of Gsk3 inhibition. Consistent with parallel operation, ESCs in LIF accommodated Esrrb deletion and remained pluripotent. These findings highlight a key role for Esrrb in regulating the naive pluripotent state and illustrate compensation among the core pluripotency factors.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
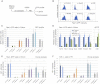
Figure 1
Identification of Tcf3 Direct Targets in Mouse ESCs (A) Flow chart illustrating the approach used to identify candidate genes that mediate self-renewal downstream of Tcf3. (B) Venn diagram showing overlap of Nanog, Oct3/4, and Tcf3 bound genes (see Experimental Procedures). (C) Venn diagram showing overlap between genes upregulated (>1.4-fold change) in Tcf3 null cells, genes upregulated in Tcf3 knockdown, and genes bound by Tcf3, Oct3/4, and Nanog. Fifty genes are identified as candidate Tcf3 direct targets. (D) Gene expression analysis of WT ESCs treated with the Gsk3 inhibitor (CH) for the indicated times (orange columns), and of Tcf3 null ESCs (blue columns). All cultures were in LIF+serum. The fold change expression relative to WT ESCs treated with vehicle (DMSO) is shown on a logarithmic scale. Mean and SD of two independent experiments is shown. (E) Gene tracks represent binding of Nanog, Oct3/4, and Tcf3 at the indicated gene loci. The x axis represents the linear sequence of genomic DNA and the y axis represents the total number of mapped reads (see Experimental Procedures). See also Figure S1.
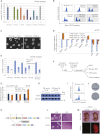
Figure 2
Esrrb Is Required to Mediate Self-Renewal Downstream of GSK3 (A) Experimental scheme for testing the functional requirement of the candidate genes identified in Figure 2. Rex1-GFPd2 cells were cultured in presence of the Gsk3 inhibitor (Chiron-N2B27) for two passages and transfected with two independent siRNAs for each candidate gene. Cells were harvested 48 hr after transfection and analyzed by flow cytometry (Rex1-GFP profile), quantitative PCR, and colony formation. (B) Rex1-GFPd2 cells were transfected with the indicated siRNAs and analyzed after 48 hr by flow cytometry. One representative plot for knockdown of each gene is shown. The dashed line indicates the threshold used to separate GFP-positive and GFP-negative cells. (C) Quantification of the flow cytometry data for Rex1-GFPd2 cells transfected with the indicated siRNAs. Columns show the number of GFP positive cells normalized to the negative control siRNA (siControl). Mean and SD of six independent experiments is shown. (D) Gene expression analysis of Rex1-GFPd2 cells transfected with two independent siRNAs targeting Esrrb or two negative control siRNAs (siControl and siGFP). GAPDH was used as endogenous control and data are normalized to the siControl sample. Mean and SD of four independent experiments is shown. (E) Clonogenicity assay on Rex1-GFPd2 cells transfected with the indicated siRNAs. Forty-eight hr after transfection, cells were replated at clonal density in 2i + LIF and stained for AP after 5 days. Columns show the number of AP+ve colonies normalized to the siControl. Mean and SD of four independent experiments is shown. (F) Clonogenicity assay of Tcf3 null cells transfected with the indicated siRNAs. Tcf3 null cells cultured in PD were transfected with the indicated siRNAs; after 48 hr they were replated at clonal density in PD and stained for alkaline phosphatase after 5 days. Columns show the number of AP+ve colonies normalized to the negative control siRNA (siControl). Mean and SD of three independent experiments is shown. See also Figures S2B–S2D.
Figure 3
Esrrb Recapitulates the Effect of GSK3 Inhibition on ESC Self-Renewal (A) Rex1-GFPd2 cells were cotransfected with pBase helper plasmid and a piggyBac vector containing Esrrb, Klf2, Nanog, Nr0b1, Tcfcp2l1, or no cDNA (PB-vector); transfected cells were selected for two passages with Hygromycin in 2i. Six hundred cells were then plated at clonal density in the basal media N2B27 with (+) or without (−) Chiron and stained for AP after 5 days. Columns show the number of AP+ve colonies. Mean and SD of three independent experiments is shown. (B) Flow cytometry analysis of Rex1-GFPd2 transfectants cultured in the indicated conditions. PB-vector cells could not be maintained for more than two passages in presence of the Mek inhibitor PD without CH, whereas PB-Klf2, PB-Nanog, and PB-Nr0b1 cells could not be maintained for more than four passages. Only PB-Esrrb cells showed robust self-renewal under these conditions, expanding continuously for more than 12 passages. (C) Phase contrast pictures of PB-vector transfected cells in 2i media and PB-Esrrb cells in presence of the Mek inhibitor PD. (D) Gene expression analysis of Rex1-GFPd2 cells transfected with either an empty vector or PB-Esrrb and cultured in the indicated conditions. Two independent PB-Esrrb transfections were carried out, generating two independent cell lines named “Rex1-GFPd2 PB-Esrrb A” and “Rex1GFPd2 PB-Esrrb B.” GAPDH was used as endogenous control and data are normalized to PB-vector cells cultured in 2i media. (E) Clonogenicity assay of Rex1-GFPd2 cells transfected with the indicated plasmids and selected for two passages. Six hundred cells were plated at clonal density in Serum media alone or with LIF and stained for AP after 5 days. Columns show the number of wholly AP+ve, mixed or wholly differentiated (AP-ve) colonies. Mean and SD of three independent experiments is shown. See also Figure S3A. (F) Rex1-GFPd2 cells in 2i were cotransfected with pBase helper plasmid and a piggyBac vector containing Esrrb-IRES-Neo (PB Esrrb IN); after 48 hr of culture in 2i, selection was applied and CH was withdrawn. Selection in low G418 (200 mg/ml) allowed isolation of transfectants expressing the Esrrb transgene at a level similar to endogenous expression in CH-treated cells. Cells were cultured for >3 passages and analyzed as indicated. (G) Gene expression analysis of Rex1GFP cells transfected with an empty vector (PB-vector) cultured in 2i, in PD for two passages, or Rex1GFP Esrrb IN cells cultured in PD for three passages. Note that in Esrrb IN cells cultured in PD (orange columns), the expression of Esrrb is maintained at levels comparable to control cells cultured in 2i (blue columns). ActinB served as an internal control. (H) Western blot of Rex1GFP cells transfected with an empty vector (PB-vector) cultured in 2i, and Rex1GFP Esrrb IN cells cultured in PD for three passages. For each sample, three different amounts of total proteins (equivalent to 50,000, 25,000, and 12,500 cells) were loaded. Note that Esrrb protein levels are comparable in the two samples. GAPDH served as a loading control. (I) Rex1GFP cells transfected with an empty vector (PB-vector) cultured in 2i for three passages, and Rex1GFP Esrrb IN cells cultured in PD for three passages have been analyzed by flow cytometry. Seventy-nine percent of Esrrb IN cells were GFP-positive. (J) Rex1GFP cells transfected with an empty vector (PB-vector) cultured in 2i for three passages, and Rex1GFP Esrrb IN cells cultured in PD for three passages have been plated at clonal density in N2B27+PD and stained for alkaline phosphatase after 5 days. As expected, PB-vector cells could not form AP+ colonies (top panel), whereas Rex1GFP Esrrb IN cells did (bottom panel). (K) Cre excisable construct used for Esrrb overexpression. (L) Representative pictures of cells transfected with the Esrrb excisable construct before (Esrrb+) and after excision (Esrrb-). Note that only Esrrb+ cells can be cultured in serum containing media without LIF. Successful excision was confirmed by PCR using transgene-specific primers (Figure S3H). (M) Esrrb excised ESCs contribute to chimeric embryos. The DsRed signal is specifically detected in the injected embryos at midgestation and not in a sibling embryo with no chimeric contribution (control).
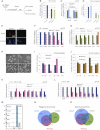
Figure 4
Esrrb Is Not Required for Self-Renewal in the Presence of LIF (A) Rex1-GFPd2 cells were cultured in N2B27 plus LIF and PD03 for two passages and transfected with two independent siRNAs targeting Esrrb or two negative control siRNAs (siControl and siGFP). Cells were harvested 48 hr after transfection and analyzed by flow cytometry (Rex1-GFP profile), quantitative PCR, and clonal analysis. (B) Rex1-GFPd2 cells were transfected with the indicated siRNAs and analyzed after 48 hr by flow cytometry. Columns show the number of GFP-positive cells normalized to the negative control siRNA (siControl). Mean and SD of three independent experiments is shown. (C) Gene expression analysis of Rex1-GFPd2 cells transfected with two independent siRNAs targeting Esrrb or two control siRNAs (siControl and siGFP). GAPDH was used as endogenous control and data are normalized to the siControl. Mean and SD of three independent experiments is shown. (D) Quantification of clonogenicity assay of Rex1-GFPd2 cells transfected with the indicated siRNAs. Forty-eight hr after transfection, cells were replated at clonal density in 2i media and stained for AP after 5 days. Columns show the number of AP+ve colonies normalized to the siControl. Mean and SD of three independent experiments is shown. (E) Immunostaining of _Esrrb_−/− and _Esrrb_fl/fl cells confirming absence of Esrrb protein in the _Esrrb_−/− cells. (F) Gene expression analysis of _Esrrb_−/− and _Esrrb_fl/fl cells cultured in LIF+Serum. Note the absence of Esrrb transcript. GAPDH was used as endogenous control and data are normalized to _Esrrb_fl/fl cells. (G) Gene expression analysis of _Nanog_−/− and Nanog+/− cells cultured in LIF+Serum. Note the absence of Nanog transcript. GAPDH was used as endogenous control and data are normalized to Nanog+/− cells. (H) Phase contrast images of _Esrrb_fl/fl and _Esrrb_−/− cells under the indicated culture conditions. (I and J) Colony forming assay on _Esrrb_fl/fl and _Esrrb_−/− (I), and _Nanog_−/− and Nanog+/− (J) cells. Cells were cultured in 2i+LIF, plated at clonal density (300 cells per well) under the indicated conditions, and stained for alkaline phosphatase after 5 days. Columns show the number of AP+ve colonies. Mean and SD of three independent experiments is shown. (K and L) _Esrrb_fl/fl and _Esrrb_−/− ESCs were cultured for 48 hr in LIF+PD (K) or CH (L) and analyzed by qPCR for the indicated pluripotency markers. GAPDH was used as endogenous control and data are normalized to Esrrbfl/fl. Mean and SD of two independent experiments is shown. (M) Gene tracks representing binding of Nanog, Oct3/4, Sox2, Tcf3, and Esrrb at the Tbx3 gene locus. The x axis represents the linear sequence of genomic DNA, and the y axis represents the total number of mapped reads. A blue box highlights a region where the five factors colocalize while the green box highlights a region bound only by Esrrb. (N) Venn diagram showing the intersection between the genomic regions bound by at least one factor among Oct3/4, Sox2, and Tcf3 (O/S/T in blue), by Nanog (in red) and by Esrrb (in green). For this diagram, only the top 5,000 ChIP-Seq peaks of each factor have been used, in order to account for differences in ChIP efficiency among different factors. See also Figure S4C. (O) Venn diagram showing the intersection between the predicted target genes bound by at least one factor among Oct3/4, Sox2, and Tcf3 (O/S/T in blue), and the predicted target genes of Nanog (in red) or Esrrb (in green). Only the top 5,000 peaks for each factor have been used to predict target genes in order to account for differences in ChIP efficiency. See also Figure S4D and Table S5.
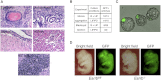
Figure 5
Esrrb Null Cells Are Pluripotent (A) _Esrrb_−/− ESCs cultured in LIF+serum were injected into the kidney capsule and produced teratocarcinomas containing tissues representative of the three germ layers (epidermis for ectoderm, kidney and striated muscle for mesoderm and gut-like epithelia for endoderm) along with undifferentiated embryonal carcinoma (EC) cells. (B) _Esrrb_−/− ESCs contribute to chimeric embryos: summary of the experiments performed with GFP-labeled _Esrrb_−/− ESCs. See also Figures S5A and S5B. (C) GFP-labeled _Esrrb_−/− ESCs cultured in either 2i+LIF or LIF/PD, were combined with 8-cell stage embryos, cultured in vitro for 48 hr, and scored for the presence of GFP-positive cells in the ICM. A representative image is shown. (D) Blastocyst injection was performed followed by embryo transfer and embryos were scored at midgestation (E12.5) for the presence of GFP-positive cells. Esrrb fl/fl served as a positive control. See also Figures S5A and S5B.
Figure 6
Mechanism of Esrrb Regulation by Tcf3 (A) Fluorescence micrographs showing immunostaining for Esrrb of WT ESCs cultured under the indicated conditions. (B) Histogram showing the distribution of Esrrb immunostaining intensity under the indicated conditions. More than 3,000 single ESCs for each condition were analyzed and divided into three categories based on staining intensity (see Experimental Procedures section). (C) Top shows that gene tracks represent binding of Tcf3 at the Esrrb gene locus. The five red boxes indicate the regions analyzed by ChIP for Tcf3. Bottom shows that ChIP for Tcf3 followed by qPCR for the indicated regions was performed in either WT or Tcf3 null cells. Enrichment over a mock ChIP is shown. Mean and SD of three independent experiments is shown. (D) Esrrb expression analysis of the indicated ESC lines, cultured in LIF/PD and treated with the Gsk3 inhibitor (CH) for 24 hr (orange columns). The fold change expression relative to LIF/PD is shown; ActinB served as an internal control. Mean and SD of two independent experiments is shown. (E) Gene expression analysis of Nanog +/− and −/− cells, cultured in LIF/PD and treated with the Gsk3 inhibitor (CH) for 8 hr or 24 hr (orange columns). GAPDH served as an internal control. Mean and SD of two independent experiments is shown. (F) Schematic representation of core pluripotency transcription factor circuit with parallel input from LIF/Stat3 and GSK3 inhibition/Tcf3 derepression. See also Figure S6.
Comment in
- Pluripotency network in embryonic stem cells: maybe Leibniz was right all along.
Zwaka TP. Zwaka TP. Cell Stem Cell. 2012 Oct 5;11(4):441-2. doi: 10.1016/j.stem.2012.09.005. Cell Stem Cell. 2012. PMID: 23040470
Similar articles
- Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation.
Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A. Wray J, et al. Nat Cell Biol. 2011 Jun 19;13(7):838-45. doi: 10.1038/ncb2267. Nat Cell Biol. 2011. PMID: 21685889 Free PMC article. - Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal.
Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Yi F, et al. Nat Cell Biol. 2011 Jun 19;13(7):762-70. doi: 10.1038/ncb2283. Nat Cell Biol. 2011. PMID: 21685894 Free PMC article. - Pramel7 mediates LIF/STAT3-dependent self-renewal in embryonic stem cells.
Casanova EA, Shakhova O, Patel SS, Asner IN, Pelczar P, Weber FA, Graf U, Sommer L, Bürki K, Cinelli P. Casanova EA, et al. Stem Cells. 2011 Mar;29(3):474-85. doi: 10.1002/stem.588. Stem Cells. 2011. PMID: 21425410 - The Art of Capturing Pluripotency: Creating the Right Culture.
Ying QL, Smith A. Ying QL, et al. Stem Cell Reports. 2017 Jun 6;8(6):1457-1464. doi: 10.1016/j.stemcr.2017.05.020. Stem Cell Reports. 2017. PMID: 28591647 Free PMC article. Review. - β-Catenin in pluripotency: adhering to self-renewal or Wnting to differentiate?
Sineva GS, Pospelov VA. Sineva GS, et al. Int Rev Cell Mol Biol. 2014;312:53-78. doi: 10.1016/B978-0-12-800178-3.00002-6. Int Rev Cell Mol Biol. 2014. PMID: 25262238 Review.
Cited by
- FoxO transcription factors actuate the formative pluripotency specific gene expression programme.
Santini L, Kowald S, Cerron-Alvan LM, Huth M, Fabing AP, Sestini G, Rivron N, Leeb M. Santini L, et al. Nat Commun. 2024 Sep 9;15(1):7879. doi: 10.1038/s41467-024-51794-9. Nat Commun. 2024. PMID: 39251582 Free PMC article. - Possible Strategies to Reduce the Tumorigenic Risk of Reprogrammed Normal and Cancer Cells.
Lin YC, Ku CC, Wuputra K, Liu CJ, Wu DC, Satou M, Mitsui Y, Saito S, Yokoyama KK. Lin YC, et al. Int J Mol Sci. 2024 May 9;25(10):5177. doi: 10.3390/ijms25105177. Int J Mol Sci. 2024. PMID: 38791215 Free PMC article. Review. - Glycolysis-Stimulated Esrrb Lactylation Promotes the Self-Renewal and Extraembryonic Endoderm Stem Cell Differentiation of Embryonic Stem Cells.
Dong Q, Zhang Q, Yang X, Nai S, Du X, Chen L. Dong Q, et al. Int J Mol Sci. 2024 Feb 26;25(5):2692. doi: 10.3390/ijms25052692. Int J Mol Sci. 2024. PMID: 38473939 Free PMC article. - Robust discovery of gene regulatory networks from single-cell gene expression data by Causal Inference Using Composition of Transactions.
Shojaee A, Huang SC. Shojaee A, et al. Brief Bioinform. 2023 Sep 22;24(6):bbad370. doi: 10.1093/bib/bbad370. Brief Bioinform. 2023. PMID: 37897702 Free PMC article. - Plakoglobin is a mechanoresponsive regulator of naive pluripotency.
Kohler TN, De Jonghe J, Ellermann AL, Yanagida A, Herger M, Slatery EM, Weberling A, Munger C, Fischer K, Mulas C, Winkel A, Ross C, Bergmann S, Franze K, Chalut K, Nichols J, Boroviak TE, Hollfelder F. Kohler TN, et al. Nat Commun. 2023 Jul 7;14(1):4022. doi: 10.1038/s41467-023-39515-0. Nat Commun. 2023. PMID: 37419903 Free PMC article.
References
- Burdon T., Stracey C., Chambers I., Nichols J., Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 1999;210:30–43. - PubMed
- Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. - PubMed
- Chen X., Xu H., Yuan P., Fang F., Huss M., Vega V.B., Wong E., Orlov Y.L., Zhang W., Jiang J. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G1100526/MRC_/Medical Research Council/United Kingdom
- G0900729/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- G1001028/MRC_/Medical Research Council/United Kingdom
- 12765/CRUK_/Cancer Research UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 100140/WT_/Wellcome Trust/United Kingdom
- G0800784/MRC_/Medical Research Council/United Kingdom
- 079249/WT_/Wellcome Trust/United Kingdom
- BB/H531527/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous