Stress-related neuropeptides and addictive behaviors: beyond the usual suspects - PubMed (original) (raw)
Review
Stress-related neuropeptides and addictive behaviors: beyond the usual suspects
Jesse R Schank et al. Neuron. 2012.
Abstract
Addictive disorders are chronic, relapsing conditions that cause extensive disease burden. Genetic factors partly account for susceptibility to addiction, but environmental factors such as stressful experiences and prolonged exposure of the brain to addictive drugs promote its development. Progression to addiction involves neuroadaptations within neurocircuitry that mediates stress responses and is influenced by several peptidergic neuromodulators. While corticotrophin releasing factor is the prototypic member of this class, recent work has identified several additional stress-related neuropeptides that play an important role in regulation of drug intake and relapse, including the urocortins, nociceptin, substance P, and neuropeptide S. Here, we review this emerging literature, discussing to what extent the properties of these neuromodulators are shared or distinct and considering their potential as drug targets.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
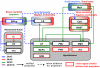
Figure 1. Ucn circuitry potentially impacting addiction-related behaviors
EWcp is the main site of Ucn1 production in brain. Ucn1 projections (blue) from this structure to the lateral septum (LS) and dorsal raphe (DR), two structures that mediate behavioral stress responses, are well established. Ucn1-immunoreactive fibers are, however, widely distributed, and the number of Ucn1-innervated brain regions is likely much greater than depicted. Projections from Ucn2 cell bodies (red) largely await characterization, while much of the forebrain Ucn3 circuitry has been mapped, and is indicated (green). Ucn1 activates both CRF1 and CRF2, receptors, while Ucn2 and 3 are CRF2R selective (see main text). CRF1R are widely distributed in the brain and are not shown; areas indicated in the figure as targets of Ucn peptides all contain CRF2 receptors to various degrees. Endogenous Ucn1 pathways originating from the EWcp mediate positively reinforcing effects of alcohol, although exogenous Ucn1 administration inhibits alcohol intake. The latter effect presumably reflects actions on targets that are innervated by endogenous Ucn2 or Ucn3 pathways, since exogenous administration of the latter two peptides inhibits alcohol intake. Endogenous Ucn2 or Ucn3 positively regulate the acute locomotor response to methamphetamine, a response that involves amygdala neurons.
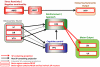
Figure 2. SP / NK1R circuitry potentially impacting addiction-related behaviors
Brain regions that receive SP projections and contain NK1Rs to varying degrees are shown. SP and NK1Rs have been shown to regulate the activity of brainstem and midbrain monoamine nuclei (NE neurons of LC, DA neurons of VTA, 5-HT neurons of DR), which have widespread projections to forebrain regions; some of these, relevant for addiction-related behaviors, are included in the current schematic. Some 5-HT neurons of the DR co-express SP, but target regions of this subset are not established; the projections that contain 5-HT potentially co-localized with SP are shown in gray. The amygdala (AMG) and hypothalamus (HYP), which regulate behavioral, autonomic and endocrine stress responses contain intrinsic SP circuits that modulate their output. The pathway from the prefrontal cortex (PFC) to the nucleus accumbens (NAC; core subregion) to the ventral pallidum (VP) is part of a proposed “final common pathway” for reinstatement of drug seeking (see main text). Medium spiny neurons (MSNs) of the nucleus accumbens (NAC) that project to the ventral pallidum (VP) and substantia nigra (SN) contain SP and activate NK1R and/or NK3R in these regions. These SP containing GABAergic projections are shown in red. Not shown is the habenula, an NK1R containing structure recently postulated to mediate important anti-reward processes; a role of SP and NK1R in these has not yet been evaluated.
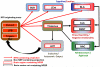
Figure 3. NPS / NPSR circuitry potentially impacting addiction-related behaviors
NPS is expressed in about 500 cells located between the Peri-LC (locus coeruleus), the lateral parabrachial nucleus and the principal sensory trigeminal nucleus, where it is largely co-expressed with Glu and CRF, respectively. NPS cells project (red arrows) to three main target clusters: the hypothalamus (HYP; grey), which regulates, basic physiological functions such as feeding and arousal; the thalamus (light brown), which integrates somatosensory imputs, as well as endocrine and autonomic responses; and a third cluster (light blue) composed of the Subiculum (SUB), Basolateral Amygdala (BLA) and Lateral Entorhinal Cortex (LEnt), involved in emotional memory. The thalamus sends non-NPS projections (black arrows) to several brain regions, including central amygdala (CeA) and bed nucleus of the stria terminalis (BNST), that are involved in emotional aspects of stress responses. The CeA as well as the nucleus accumbens (NAC) also receive heavy non-NPS projection from the basolateral amygdala (BLA) and cortical areas (not depicted in this schematic) that integrate cognitive function with emotional stress and reward processing. NPS neurotransmission is located upstream of these pathways, and can therefore have complex effects on drug seeking and taking, that impact both negatively and positively reinforced aspects of these behaviors.
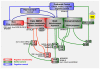
Figure 4. An integrated view
Key nodes in circuitry that drives drug seeking and self-administration [adapted from (Koob and Volkow, 2010)] modulated by Ucn family peptides and CRF2R, SP and NK1R, NPS and NPSR, and N/OFQ and NOPR systems. Nodes at which modulation is likely to occur through effects on stress-reactivity and negative emotionality are shown in red; those at which modulation is likely to influence appetitive or approach-related mechanisms are depicted in green. The figures shows that the systems discussed in this review can impact addiction-related behaviors at multiple sites. Their impact is likely to vary with genetic factors that influence the functional activity of the respective system, as well as drug exposure history of the individual, and concomitant neuroadaptations. Although many effects on drug seeking and taking have been described following manipulation of these systems, their complexity suggests that extensive research will be required to properly assess their potential as therapeutic targets, and to define patient characteristics most likely predictive of efficacy.
Similar articles
- Neuropeptide modulation of addiction: Focus on galanin.
Genders SG, Scheller KJ, Djouma E. Genders SG, et al. Neurosci Biobehav Rev. 2020 Mar;110:133-149. doi: 10.1016/j.neubiorev.2018.06.021. Epub 2018 Jun 24. Neurosci Biobehav Rev. 2020. PMID: 29949733 Review. - Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin.
Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Martin-Fardon R, et al. Brain Res. 2010 Feb 16;1314:145-61. doi: 10.1016/j.brainres.2009.12.027. Epub 2009 Dec 21. Brain Res. 2010. PMID: 20026088 Free PMC article. Review. - Corticotropin releasing factor: a key role in the neurobiology of addiction.
Zorrilla EP, Logrip ML, Koob GF. Zorrilla EP, et al. Front Neuroendocrinol. 2014 Apr;35(2):234-44. doi: 10.1016/j.yfrne.2014.01.001. Epub 2014 Jan 20. Front Neuroendocrinol. 2014. PMID: 24456850 Free PMC article. Review. - From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use?
McGregor IS, Callaghan PD, Hunt GE. McGregor IS, et al. Br J Pharmacol. 2008 May;154(2):358-68. doi: 10.1038/bjp.2008.132. Br J Pharmacol. 2008. PMID: 18475254 Free PMC article. Review. - Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin.
Bisagno V, Cadet JL. Bisagno V, et al. Behav Pharmacol. 2014 Sep;25(5-6):445-57. doi: 10.1097/FBP.0000000000000049. Behav Pharmacol. 2014. PMID: 24949572 Free PMC article. Review.
Cited by
- The nociceptin/orphanin FQ receptor agonist SR-8993 as a candidate therapeutic for alcohol use disorders: validation in rat models.
Aziz AM, Brothers S, Sartor G, Holm L, Heilig M, Wahlestedt C, Thorsell A. Aziz AM, et al. Psychopharmacology (Berl). 2016 Oct;233(19-20):3553-63. doi: 10.1007/s00213-016-4385-8. Epub 2016 Aug 11. Psychopharmacology (Berl). 2016. PMID: 27515665 Free PMC article. - Heat Stress Biomarker Amino Acids and Neuropeptide Afford Thermotolerance in Chicks.
Chowdhury VS. Chowdhury VS. J Poult Sci. 2019 Jan 25;56(1):1-11. doi: 10.2141/jpsa.0180024. J Poult Sci. 2019. PMID: 32055190 Free PMC article. Review. - The neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental study.
Kwako LE, George DT, Schwandt ML, Spagnolo PA, Momenan R, Hommer DW, Diamond CA, Sinha R, Shaham Y, Heilig M. Kwako LE, et al. Psychopharmacology (Berl). 2015 Jan;232(1):295-304. doi: 10.1007/s00213-014-3665-4. Epub 2014 Jul 18. Psychopharmacology (Berl). 2015. PMID: 25030801 Free PMC article. Clinical Trial. - Emerging targets for addiction neuropharmacology: From mechanisms to therapeutics.
Ubaldi M, Cannella N, Ciccocioppo R. Ubaldi M, et al. Prog Brain Res. 2016;224:251-84. doi: 10.1016/bs.pbr.2015.07.018. Epub 2015 Nov 26. Prog Brain Res. 2016. PMID: 26822362 Free PMC article. Review. - Rostromedial tegmental nucleus nociceptin/orphanin FQ (N/OFQ) signaling regulates anxiety- and depression-like behaviors in alcohol withdrawn rats.
Li W, Ren Z, Tang Y, Fu Y, Sun S, Ding R, Hou J, Mai Y, Zhan B, Zhu Y, Zuo W, Ye JH, Fu R. Li W, et al. Neuropsychopharmacology. 2023 May;48(6):908-919. doi: 10.1038/s41386-022-01482-3. Epub 2022 Nov 3. Neuropsychopharmacology. 2023. PMID: 36329156 Free PMC article.
References
- Alcaro A, Panksepp J. The SEEKING mind: primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci. Biobehav. Rev. 2011;35:1805–1820. - PubMed
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur. J. Neurosci. 2004;20:1613–1623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DA007262/DA/NIDA NIH HHS/United States
- AA016647/AA/NIAAA NIH HHS/United States
- Z01 AA000487/ImNIH/Intramural NIH HHS/United States
- R01 AA017447/AA/NIAAA NIH HHS/United States
- F31 AA021023/AA/NIAAA NIH HHS/United States
- U01 AA016647/AA/NIAAA NIH HHS/United States
- AA021023/AA/NIAAA NIH HHS/United States
- AA019793/AA/NIAAA NIH HHS/United States
- AA017447/AA/NIAAA NIH HHS/United States
- R01 AA019793/AA/NIAAA NIH HHS/United States
- R01 AA014351/AA/NIAAA NIH HHS/United States
- AA01760/AA/NIAAA NIH HHS/United States
- Z01 AA000487-03/ImNIH/Intramural NIH HHS/United States
- AA014351/AA/NIAAA NIH HHS/United States
- R37 AA017447/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical