Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression - PubMed (original) (raw)
Review
Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression
Jong Hun Lee et al. Pharmacol Ther. 2013 Feb.
Abstract
Reactive metabolites from carcinogens and oxidative stress can drive genetic mutations, genomic instability, neoplastic transformation, and ultimately carcinogenesis. Numerous dietary phytochemicals in vegetables/fruits have been shown to possess cancer chemopreventive effects in both preclinical animal models and human epidemiological studies. These phytochemicals could prevent the initiation of carcinogenesis via either direct scavenging of reactive oxygen species/reactive nitrogen species (ROS/RNS) or, more importantly, the induction of cellular defense detoxifying/antioxidant enzymes. These defense enzymes mediated by Nrf2-antioxidative stress and anti-inflammatory signaling pathways can contribute to cellular protection against ROS/RNS and reactive metabolites of carcinogens. In addition, these compounds would kill initiated/transformed cancer cells in vitro and in in vivo xenografts via diverse anti-cancer mechanisms. These mechanisms include the activation of signaling kinases (e.g., JNK), caspases and the mitochondria damage/cytochrome c pathways. Phytochemicals may also have anti-cancer effects by inhibiting the IKK/NF-κB pathway, inhibiting STAT3, and causing cell cycle arrest. In addition, other mechanisms may include epigenetic alterations (e.g., inhibition of HDACs, miRNAs, and the modification of the CpG methylation of cancer-related genes). In this review, we will discuss: the current advances in the study of Nrf2 signaling; Nrf2-deficient tumor mouse models; the epigenetic control of Nrf2 in tumorigenesis and chemoprevention; Nrf2-mediated cancer chemoprevention by naturally occurring dietary phytochemicals; and the mutation or hyper-expression of the Nrf2-Keap1 signaling pathway in advanced tumor cells. The future development of dietary phytochemicals for chemoprevention must integrate in vitro signaling mechanisms, relevant biomarkers of human diseases, and combinations of different phytochemicals and/or non-toxic therapeutic drugs, including NSAIDs.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Fig. 1
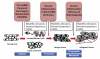
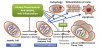
Schematic representation of multi-stage carcinogenesis. Exposure to intrinsic/extrinsic factors, including various toxic chemicals, oncogenes, viruses (e.g., HBV, hepatitis B virus), ROS/RNS, and inflammation, can resulting in genetic mutations and/or epigenetic alterations that cause the initiation of carcinogenesis in normal cells. The initiated cells and non-neoplastic cancer stem/progenitor cells can first progress to benign tumors, which would be amendable with surgery, radiation and or chemotherapy, if detected early, with subsequent progression to advanced/metastasized/malignant/drug-resistant tumors due to the prolonged effects of chronic inflammation, various irritants and aberrant hormones.
Fig. 2
MAPK signaling transduction in mammalian cells. Activation of MAPK is regulated by hierarchical cascade known as the MAPK module. Mitogens such as growth factors, cytokines, and environmental stress typically activate MAP3Ks, subsequently resulting in phosphorylation of MAP2Ks. Phosphorylated MAP2Ks activate terminal MAPKs such as Erk1/2, p38, and JNK/SAPK through phosphorylating specific threonine (T) and tyrosine (Y) residues of T-X-Y motif. Several downstream targets of MAPKs including p90RSK and transcription factors such as Elk-1, MEF-2, ATF-2, and c-JUN are specifically activated and resulted in subsequent diverse cellular events, including cell survival, proliferation, differentiation, and apoptosis. The exposure to chemopreventive dietary phytochemicals can activate MAPK pathway, leading to gene expression, apoptotic cell death and differentiation of cancer cells.
Fig. 3
The impact of dietary phytochemicals on the regulation of Nrf2-dependent pharmacogenomics. Both Nrf2 KO and WT mice were treated with dietary phytochemicals or with vehicle (control group). DNA was extracted from the organ of interest, such as the liver, prostate, or small intestine, and then hybridized to a DNA microarray. Through a comparison of the compound-treated Nrf2 KO group vs. the treated WT and non-treated Nrf2 KO groups, a large amount of Nrf2-dependant compound-induced genes was found (Thimmulappa et al., 2002; Shen et al., 2005; Hu et al., 2006a, 2006b; Nair et al., 2006; Shen et al., 2006; Barve et al., 2008; Wu et al., 2011), and some of the representative groups of genes are presented.
Fig. 4
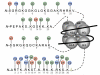
Histone tails affecting chromatin modification. Chromatin modifications usually appear at the amino acids in the N-terminal tails of histones (H2A, H2B, H3, H4), which provide the site for a wide range of posttranslational modifications. Various enzymes, such as histone acetyltransferases (HATs), histone methyltransferases (HMTs), histone deacetylases (HDACs), and histone demethylases (HDMs), are involved in these modifications, which cause covalent changes at the marked amino acids. ac: acetyl group, me: methyl group, ph: phosphate group. Modified from (Bhaumik et al., 2007).
Fig. 5
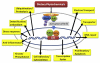
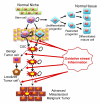
Potential differences between cancer stem cells (CSCs) and tumor cells. In normal tissues, stem cells maintain self-renewal ability ( ) by interacting with the tissue stroma (niche = green). Within the normal niche, stem cells generate progenitor cells that differentiate into mature cells. During cell division, the replication potential of daughter cells is decreased at each step. If oxidative stress and inflammation stimulate the niche, it could provide stem cells with mutation signals. In response to the mutation signals, stem cell may acquire a tumorigenic capacity and result in CSCs (star-shaped red). Undifferentiated progenitor cells (blue diamond) may also become CSC-like cells driven by the mutation signals. While CSCs proliferate within tumors, they may give a rise to all types of tumor cells at each stage of tumor progression. Benign and localized tumor cells can be potentially (dashed arrow) generated from CSCs. Advanced metastasized and malignant tumor can be formed driven by further mutational signals including additional epigenetic modifications and genetic mutations. In the context of CSC hypothesis, the subset of tumor cells containing CSCs may grow to tumor in a manner that is hierarchical and heterogeneous. In contrast, non-tumorigenic cancer cell may not form tumor successfully.
) by interacting with the tissue stroma (niche = green). Within the normal niche, stem cells generate progenitor cells that differentiate into mature cells. During cell division, the replication potential of daughter cells is decreased at each step. If oxidative stress and inflammation stimulate the niche, it could provide stem cells with mutation signals. In response to the mutation signals, stem cell may acquire a tumorigenic capacity and result in CSCs (star-shaped red). Undifferentiated progenitor cells (blue diamond) may also become CSC-like cells driven by the mutation signals. While CSCs proliferate within tumors, they may give a rise to all types of tumor cells at each stage of tumor progression. Benign and localized tumor cells can be potentially (dashed arrow) generated from CSCs. Advanced metastasized and malignant tumor can be formed driven by further mutational signals including additional epigenetic modifications and genetic mutations. In the context of CSC hypothesis, the subset of tumor cells containing CSCs may grow to tumor in a manner that is hierarchical and heterogeneous. In contrast, non-tumorigenic cancer cell may not form tumor successfully.
Fig. 6
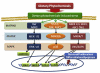
Cancer chemoprevention strategy using dietary phytochemicals and non-toxic therapeutic drugs. Oxidative stress, inflammation and reactive intermediates of carcinogens can cause genetic mutations and epigenetic alterations. Through the promotion/progression stages, initiated cells become advanced/metastatic tumor cells. Applying dietary phytochemicals at the early stage of carcinogenesis may block further development of carcinogenesis. Treatment with dietary phytochemicals and/or relatively non-toxic therapeutic drugs on cancer cells may induce positive results, including autophagy, cell cycle arrest, apoptosis, and differentiation, and may block tumor development.
Similar articles
- A perspective on dietary phytochemicals and cancer chemoprevention: oxidative stress, nrf2, and epigenomics.
Su ZY, Shu L, Khor TO, Lee JH, Fuentes F, Kong AN. Su ZY, et al. Top Curr Chem. 2013;329:133-62. doi: 10.1007/128_2012_340. Top Curr Chem. 2013. PMID: 22836898 Free PMC article. Review. - Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells.
Gopalakrishnan A, Tony Kong AN. Gopalakrishnan A, et al. Food Chem Toxicol. 2008 Apr;46(4):1257-70. doi: 10.1016/j.fct.2007.09.082. Epub 2007 Sep 15. Food Chem Toxicol. 2008. PMID: 17950513 Review. - Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals.
Qin S, Hou DX. Qin S, et al. Mol Nutr Food Res. 2016 Aug;60(8):1731-55. doi: 10.1002/mnfr.201501017. Epub 2016 Jun 1. Mol Nutr Food Res. 2016. PMID: 27523917 Review. - Cancer chemoprevention by phytochemicals: potential molecular targets, biomarkers and animal models.
Kwon KH, Barve A, Yu S, Huang MT, Kong AN. Kwon KH, et al. Acta Pharmacol Sin. 2007 Sep;28(9):1409-21. doi: 10.1111/j.1745-7254.2007.00694.x. Acta Pharmacol Sin. 2007. PMID: 17723174 Review.
Cited by
- The Epigenetic Impact of Cruciferous Vegetables on Cancer Prevention.
Royston KJ, Tollefsbol TO. Royston KJ, et al. Curr Pharmacol Rep. 2015 Feb 1;1(1):46-51. doi: 10.1007/s40495-014-0003-9. Curr Pharmacol Rep. 2015. PMID: 25774338 Free PMC article. - Role of epigenetic modifications in luminal breast cancer.
Abdel-Hafiz HA, Horwitz KB. Abdel-Hafiz HA, et al. Epigenomics. 2015 Aug;7(5):847-62. doi: 10.2217/epi.15.10. Epub 2015 Feb 17. Epigenomics. 2015. PMID: 25689414 Free PMC article. Review. - Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization.
Lingua G, Bona E, Manassero P, Marsano F, Todeschini V, Cantamessa S, Copetta A, D'Agostino G, Gamalero E, Berta G. Lingua G, et al. Int J Mol Sci. 2013 Aug 6;14(8):16207-25. doi: 10.3390/ijms140816207. Int J Mol Sci. 2013. PMID: 23924942 Free PMC article. - Quercetin Impact in Pancreatic Cancer: An Overview on Its Therapeutic Effects.
Asgharian P, Tazehkand AP, Soofiyani SR, Hosseini K, Martorell M, Tarhriz V, Ahangari H, Cruz-Martins N, Sharifi-Rad J, Almarhoon ZM, Ydyrys A, Nurzhanyat A, Yessenbekova A, Cho WC. Asgharian P, et al. Oxid Med Cell Longev. 2021 Nov 3;2021:4393266. doi: 10.1155/2021/4393266. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34777687 Free PMC article. Review. - Characterization of Novel α-Mangostin and Paeonol Derivatives With Cancer-Selective Cytotoxicity.
Nunna S, Huang YP, Rasa M, Krepelova A, Annunziata F, Adam L, Käppel S, Hsu MH, Neri F. Nunna S, et al. Mol Cancer Ther. 2022 Feb;21(2):257-270. doi: 10.1158/1535-7163.MCT-20-0787. Epub 2021 Nov 17. Mol Cancer Ther. 2022. PMID: 34789561 Free PMC article.
References
- Afshari CA, Nuwaysir EF, Barrett JC. Application of complementary DNA microarray technology to carcinogen identification, toxicology, and drug safety evaluation. Cancer Res. 1999;59:4759–4760. - PubMed
- Alam J, Wicks C, Stewart D, Gong P, Touchard C, Otterbein S, et al. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275:27694–27702. - PubMed
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA094828/CA/NCI NIH HHS/United States
- R01 CA152826/CA/NCI NIH HHS/United States
- R01-CA118947/CA/NCI NIH HHS/United States
- R01AT007065/AT/NCCIH NIH HHS/United States
- P30 ES005022/ES/NIEHS NIH HHS/United States
- R01-CA152826/CA/NCI NIH HHS/United States
- R01 CA073674/CA/NCI NIH HHS/United States
- R01 CA118947/CA/NCI NIH HHS/United States
- R01-CA94828/CA/NCI NIH HHS/United States
- R01-CA73674/CA/NCI NIH HHS/United States
- R01 AT007065/AT/NCCIH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous