Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine β-hydroxylase knockout mice - PubMed (original) (raw)
Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine β-hydroxylase knockout mice
Frances Y Hu et al. Anesthesiology. 2012 Nov.
Abstract
Background: Multiple lines of evidence suggest that the adrenergic system can modulate sensitivity to anesthetic-induced immobility and anesthetic-induced hypnosis as well. However, several considerations prevent the conclusion that the endogenous adrenergic ligands norepinephrine and epinephrine alter anesthetic sensitivity.
Methods: Using dopamine β-hydroxylase knockout (Dbh) mice genetically engineered to lack the adrenergic ligands and their siblings with normal adrenergic levels, we test the contribution of the adrenergic ligands upon volatile anesthetic induction and emergence. Moreover, we investigate the effects of intravenous dexmedetomidine in adrenergic-deficient mice and their siblings using both righting reflex and processed electroencephalographic measures of anesthetic hypnosis.
Results: We demonstrate that the loss of norepinephrine and epinephrine and not other neuromodulators co-packaged in adrenergic neurons is sufficient to cause hypersensitivity to induction of volatile anesthesia. However, the most profound effect of adrenergic deficiency is retarding emergence from anesthesia, which takes two to three times as long in Dbh mice for sevoflurane, isoflurane, and halothane. Having shown that Dbh mice are hypersensitive to volatile anesthetics, we further demonstrate that their hypnotic hypersensitivity persists at multiple doses of dexmedetomidine. Dbh mice exhibit up to 67% shorter latencies to loss of righting reflex and up to 545% longer durations of dexmedetomidine-induced general anesthesia. Central rescue of adrenergic signaling restores control-like dexmedetomidine sensitivity. A novel continuous electroencephalographic analysis illustrates that the longer duration of dexmedetomidine-induced hypnosis is not due to a motor confound, but occurs because of impaired anesthetic emergence.
Conclusions: Adrenergic signaling is essential for normal emergence from general anesthesia. Dexmedetomidine-induced general anesthesia does not depend on inhibition of adrenergic neurotransmission.
Figures
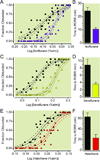
Fig.1
Adrenergic-deficient mice exhibit volatile anesthetic hypersensitivity relative to sibling controls with normal levels of norepinephrine/epinephrine. Symbols respectively depict the fraction of Dbh+/− (n=10, triangles) and _Dbh_−/− (n=12, circles) mice that exhibit a loss of righting reflex (LORR) at each specified anesthetic dose for (A) isoflurane, (C) sevoflurane, and (E) halothane. Solid lines denote the best-fit curves with dashed lines showing 95% confidence interval bracketing the best-fit curves. Bars represent mean ± SEM time lapsed from the termination of anesthetic exposure (shown in A, C, E) until the return of righting reflex (RORR) for _Dbh_−/− (black) and Dbh+/− (colored) mice for (B) isoflurane, (D) sevoflurane, and (F) halothane. Effects of genotype are significant F1,236=46.7, p < 0.0001. Atm: atmosphere; Dbh: dopamine β-hydroxylase.
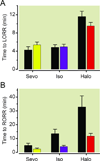
Fig. 2
Delayed emergence in _Dbh_−/− mice is not due to a relative volatile anesthetic overdose. (A) Time to LORR in _Dbh_−/− and Dbh+/− mice when each group is exposed to their respective ED95 dose for eliciting LORR. (B) Time until RORR in _Dbh_−/− (black) and Dbh+/− (colored) mice following a 2 hour exposure to each group’s respective volatile anesthetic ED95 (n=12/group). There is a significant effect of genotype on emergence F1,66=12.33, p < 0.0003, but not upon induction F1,66=0.14, p=0.7132. Bars show mean ± SEM; LORR: loss of righting reflex; RORR: return of righting reflex; Sevo: sevoflurane; Iso: isoflurane; Halo: halothane; Dbh: dopamine β-hydroxylase.
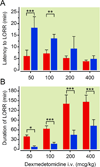
Fig. 3
Loss of adrenergic ligands is sufficient to cause hypersensitivity to dexmedetomidine as assessed by LORR. _Dbh_−/− mice illustrate hypersensitivity relative to Dbh+/− mice in response to varying intravenous doses of dexmedetomidine. (A) Latency to loss of righting reflex and (B) Duration of LORR in _Dbh_−/− (red) and Dbh+/− (blue) mice (n=5–6/group). Significant genotypic effects were found for both latency to F1,35=53.40, p < 0.0001 and duration of F1,35=158.77, p < 0.0001 dexmedetomidine-induced hypnosis. * p < 0.05, ** p < 0.01, and *** p < 0.001 relative to Dbh+/− mouse latency to LORR or duration of LORR times. Bars show mean ± SEM; LORR: loss of righting reflex; Dbh: dopamine β-hydroxylase.
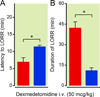
Fig. 4
CNS-specific rescue of norepinephrine and ephinephrine in adrenergic-deficient mice restores duration of dexmedetomidine-induced hypnosis to control levels. Both the latency to LORR following 50 µg/kg of intravenous dexmedetomidine (A) and the duration of LORR (B) are rescued in _Dbh_−/− mice receiving L-threo-3.4-dihydroxyphenylserine and benserazide rescue treatment (blue) compared to _Dbh_−/− mice that received vehicle treatment (red) (n=4/group). * p < 0.05. Bars show mean ± SEM; CNS: central nervous system; LORR: loss of righting reflex; Dbh: dopamine β-hydroxylase.
Fig. 5
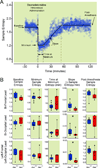
_Dbh_−/− mice show delayed emergence from dexmedetomidine relative to Dbh+/− mice using a motor-independent electroencephalographic measure. (A) Panel shows a characteristic segmental best-fit (solid line) analysis of sample entropy values calculated from raw electroencephalogram in a control mouse before, during, and after intravenous dexmedetomidine. Raw sample entropy values (circles) were calculated from the electroencephalogram, which were fit with five linear segments to approximate five variables as labeled. Dexmedetomidine was administered intravenously at time=0 as denoted by the arrow. (B) Box plots illustrate sample entropy values along with lower quartile, upper quartile, group minimum and group maximum for pre-drug baseline wakefulness, minimum entropy level, the duration of time at the minimum entropy level, the slope of sample entropy recovery that defines emergence, and sample entropy post-dexmedetomidine emergence for Dbh+/− (n=12) and _Dbh_−/− (n=8) mice as computed from right frontal-left frontal, right occipital-left occipital, and left frontal-left occipital leads. * denotes p < 0.05 in _Dbh_−/− mice relative to Dbh+/− mice. Dbh: dopamine β-hydroxylase.
Fig 6
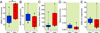
Adrenergic-deficient mice show no significant differences from sibling controls with respect to motor tone following intravenous dexmedetomidine administration. Box plots depict integrated electromyographic intensity (normalized to percentage of maximum during wakefulness) along with lower quartile, upper quartile, group minimum, and group maximum for (A) pre-drug baseline wakefulness, (B) minimum integrated electromyogram values, (C) the duration of time at the minimum integrated electromyogram values, (D) the slope of integrated electromyogram recovery, and (E) stabilized integrated electromyogram values post-dexmedetomidine emergence in Dbh+/− (n=12) and _Dbh_−/− (n=8) mice. * denotes p < 0.05 in _Dbh_−/− mice relative to Dbh+/− mice. iEMG: integrated electromyogram; Dbh: dopamine β-hydroxylase.
Comment in
- Noradrenergic trespass in anesthetic and sedative states.
Sanders RD, Maze M. Sanders RD, et al. Anesthesiology. 2012 Nov;117(5):945-7. doi: 10.1097/ALN.0b013e3182700c93. Anesthesiology. 2012. PMID: 23042229 No abstract available.
Similar articles
- Noradrenergic trespass in anesthetic and sedative states.
Sanders RD, Maze M. Sanders RD, et al. Anesthesiology. 2012 Nov;117(5):945-7. doi: 10.1097/ALN.0b013e3182700c93. Anesthesiology. 2012. PMID: 23042229 No abstract available. - Mitochondrial Function in Astrocytes Is Essential for Normal Emergence from Anesthesia in Mice.
Ramadasan-Nair R, Hui J, Itsara LS, Morgan PG, Sedensky MM. Ramadasan-Nair R, et al. Anesthesiology. 2019 Mar;130(3):423-434. doi: 10.1097/ALN.0000000000002528. Anesthesiology. 2019. PMID: 30707122 Free PMC article. - Effects of dexmedetomidine on sevoflurane requirement for 50% excellent tracheal intubation in children: a randomized, double-blind comparison.
He L, Wang X, Zheng S. He L, et al. Paediatr Anaesth. 2014 Sep;24(9):987-93. doi: 10.1111/pan.12430. Epub 2014 May 14. Paediatr Anaesth. 2014. PMID: 24823715 Review. - Role of signal transduction in anesthetic action. Alpha 2 adrenergic agonists.
Maze M, Regan JW. Maze M, et al. Ann N Y Acad Sci. 1991;625:409-22. doi: 10.1111/j.1749-6632.1991.tb33868.x. Ann N Y Acad Sci. 1991. PMID: 1711813 Review.
Cited by
- Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia.
Vazey EM, Aston-Jones G. Vazey EM, et al. Proc Natl Acad Sci U S A. 2014 Mar 11;111(10):3859-64. doi: 10.1073/pnas.1310025111. Epub 2014 Feb 24. Proc Natl Acad Sci U S A. 2014. PMID: 24567395 Free PMC article. - Adrenergic mechanisms of absence status epilepticus.
Sitnikova E. Sitnikova E. Front Neurol. 2023 Nov 22;14:1298310. doi: 10.3389/fneur.2023.1298310. eCollection 2023. Front Neurol. 2023. PMID: 38073616 Free PMC article. Review. - Regulation of I1-imidazoline receptors on the sedation effect of dexmedetomidine in mice.
Han X, Yang ZF, Zhao TY, Lu GY, Wang ZY, Wu N, Li J, Li F. Han X, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug;397(8):5927-5937. doi: 10.1007/s00210-024-02991-2. Epub 2024 Feb 16. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38363351 - Isoflurane-induced inactivation of CREB through histone deacetylase 4 is responsible for cognitive impairment in developing brain.
Sen T, Sen N. Sen T, et al. Neurobiol Dis. 2016 Dec;96:12-21. doi: 10.1016/j.nbd.2016.08.005. Epub 2016 Aug 17. Neurobiol Dis. 2016. PMID: 27544482 Free PMC article. - Historical and Modern Evidence for the Role of Reward Circuitry in Emergence.
Heshmati M, Bruchas MR. Heshmati M, et al. Anesthesiology. 2022 Jun 1;136(6):997-1014. doi: 10.1097/ALN.0000000000004148. Anesthesiology. 2022. PMID: 35362070 Free PMC article. Review.
References
- Miller RD, Way WL, Eger EI., 2nd The effects of alpha-methyldopa, reserpine, guanethidine, and iproniazid on minimum alveolar anesthetic requirement (MAC) Anesthesiology. 1968;29:1153–1158. - PubMed
- Johnston RR, White PF, Eger EI., 2nd Comparative effects of dextroamphetamine and reserpine on halothane and cyclopropane anesthetic requirements. Anesth Analg. 1975;54:655–659. - PubMed
- Johnston RR, Way WL, Miller RD. Alteration of anesthetic requirement by amphetamine. Anesthesiology. 1972;36:357–363. - PubMed
- Doze VA, Chen BX, Maze M. Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central alpha-2 adrenoceptors. Anesthesiology. 1989;71:75–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous