Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies - PubMed (original) (raw)
Clinical Trial
. 2013 Jan 1;31(1):88-94.
doi: 10.1200/JCO.2012.42.7906. Epub 2012 Oct 8.
Joseph J Buggy, Jeff P Sharman, Sonali M Smith, Thomas E Boyd, Barbara Grant, Kathryn S Kolibaba, Richard R Furman, Sara Rodriguez, Betty Y Chang, Juthamas Sukbuntherng, Raquel Izumi, Ahmed Hamdy, Eric Hedrick, Nathan H Fowler
Affiliations
- PMID: 23045577
- PMCID: PMC5505166
- DOI: 10.1200/JCO.2012.42.7906
Clinical Trial
Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies
Ranjana H Advani et al. J Clin Oncol. 2013.
Abstract
Purpose: Survival and progression of mature B-cell malignancies depend on signals from the B-cell antigen receptor, and Bruton tyrosine kinase (BTK) is a critical signaling kinase in this pathway. We evaluated ibrutinib (PCI-32765), a small-molecule irreversible inhibitor of BTK, in patients with B-cell malignancies.
Patients and methods: Patients with relapsed or refractory B-cell lymphoma and chronic lymphocytic leukemia received escalating oral doses of ibrutinib. Two schedules were evaluated: one, 28 days on, 7 days off; and two, once-daily continuous dosing. Occupancy of BTK by ibrutinib in peripheral blood was monitored using a fluorescent affinity probe. Dose escalation proceeded until either the maximum-tolerated dose (MTD) was achieved or, in the absence of MTD, until three dose levels above full BTK occupancy by ibrutinib. Response was evaluated every two cycles.
Results: Fifty-six patients with a variety of B-cell malignancies were treated over seven cohorts. Most adverse events were grade 1 and 2 in severity and self-limited. Dose-limiting events were not observed, even with prolonged dosing. Full occupancy of the BTK active site occurred at 2.5 mg/kg per day, and dose escalation continued to 12.5 mg/kg per day without reaching MTD. Pharmacokinetic data indicated rapid absorption and elimination, yet BTK occupancy was maintained for at least 24 hours, consistent with the irreversible mechanism. Objective response rate in 50 evaluable patients was 60%, including complete response of 16%. Median progression-free survival in all patients was 13.6 months.
Conclusion: Ibrutinib, a novel BTK-targeting inhibitor, is well tolerated, with substantial activity across B-cell histologies.
Trial registration: ClinicalTrials.gov NCT00849654.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Fig 1.
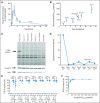
Pharmacokinetic and pharmacodynamic studies of ibrutinib. (A) Mean plasma concentration of ibrutinib over time at the 560-mg per day dose and (B) relationship between plasma area under the concentration-time curve (AUC) and dose are shown. Error bars represent SEM; arrow indicates the 560-mg fixed dose, which corresponded to a mean dose of 7.22 mg/kg per day (range, 5.6 to 9.3 mg/kg per day) based on actual patient body weight. (C) Representative probe assay data from peripheral blood mononuclear cell (PBMC) samples collected from patient with chronic lymphocytic leukemia from dose-level cohort IV (8.3 mg/kg per day). The probe (fluorescently tagged derivative of ibrutinib) binds to Bruton tyrosine kinase (BTK; indicated by arrow) with remarkably little specific labeling of other proteins. Total BTK and actin protein levels in each sample are shown to normalize protein in each lane. The BTK-bound fluorescent probe is unable to bind BTK in the samples obtained after drug administration because of occupancy of the binding site by ibrutinib. (D) Normalized intensity of probe labeling is plotted, and the lower limit of detection (LLOD) for this gel is indicated. (E) Comparison of the degree of BTK occupancy by ibrutinib at each dose-level cohort in the study. Dots represent a single BTK occupancy measurement using the probe assay; line indicates median percentage occupancy; asterisks denote P < .001 (analysis of variance). These data suggest that full BTK occupancy was generally achieved in patients at doses ≥ 2.5 mg/kg. (F) Pharmacokinetic and pharmacodynamic relationship of BTK active-site occupancy and ibrutinib exposure. Samples of PBMCs were collected predose and at 4 and 24 hours after administration of ibrutinib for each patient. Data represent the percentage of BTK occupancy before dosing and averaged between 4 and 24 hours postdose for each patient in each group. These values are plotted against the drug exposure (AUC_0-24_) achieved in the patient after administration of ibrutinib on day 1. Line represents the line of best fit of a simple maximum-effect model to the data. Analysis of pharmacokinetic and pharmacodynamic profiles on day 1 showed that BTK active-site occupancy was saturated or near saturated (> 95%) at AUC values ≥ 160 ng × h/mL.
Fig 2.
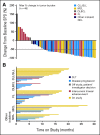
Antitumor response in all evaluable patients. (A) Best responses in the 48 patients evaluated by computed tomography scan for change from baseline in the sum of the largest diameter of each target lesion; negative values indicate tumor shrinkage. (B) Time on study for all 56 patients grouped by histology; bars describe the reason for patient discontinuation. CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; DLT, dose-limiting toxicity; FL, follicular lymphoma; NHL, non-Hodgkin lymphoma; MCL, mantle-cell lymphoma; SLL, small lymphocytic lymphoma; SPD, sum of product of greatest diameters.
Fig A1.
Serum immunoglobulin (Ig) levels of patients treated with ibrutinib over 12 cycles. Total (A) IgG and (B) IgM levels from cycles one (predose; n = 54), three (n = 18), six (n = 28), nine (n = 10), and 12 (n = 7) were determined for patients and converted to percentage by using the predose level as 100%; shown as mean plus SEM in each bar graph.
Fig A2.
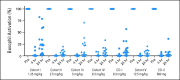
Pharmacodynamic activity of ibrutinib-treated patients in the various cohorts as determined by percentage of CD63+ basophils on α–immunoglobulin E stimulation of whole blood collected at 4 (day 1, day 8) and 24 hours (day 1, day 8, day 15, day 29) after drug administration compared with predose baseline (set at 100%). Patients with < 10% CD63+ expression at baseline after stimulation were excluded.
Fig A3.
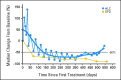
Pattern of lymphocytosis seen in patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Relationship between absolute lymphocyte count (ALC) and the sum of product of greatest diameters (SPD) in patients with CLL or SLL (n = 16). Median percentage change from baseline is shown. Sawtooth pattern suggests a transient reversal of treatment-related lymphocytosis during the 7-days-off-drug period.
Similar articles
- Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL.
Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, Harrup R, Johnston PB, Marlton P, Munoz J, Seymour JF, Simpson D, Tedeschi A, Elstrom R, Yu Y, Tang Z, Han L, Huang J, Novotny W, Wang L, Roberts AW. Tam CS, et al. Blood. 2019 Sep 12;134(11):851-859. doi: 10.1182/blood.2019001160. Epub 2019 Jul 24. Blood. 2019. PMID: 31340982 Free PMC article. Clinical Trial. - A phase 1 clinical trial of the selective BTK inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies.
Walter HS, Rule SA, Dyer MJ, Karlin L, Jones C, Cazin B, Quittet P, Shah N, Hutchinson CV, Honda H, Duffy K, Birkett J, Jamieson V, Courtenay-Luck N, Yoshizawa T, Sharpe J, Ohno T, Abe S, Nishimura A, Cartron G, Morschhauser F, Fegan C, Salles G. Walter HS, et al. Blood. 2016 Jan 28;127(4):411-9. doi: 10.1182/blood-2015-08-664086. Epub 2015 Nov 5. Blood. 2016. PMID: 26542378 Free PMC article. Clinical Trial. - Ibrutinib for the treatment of chronic lymphocytic leukemia and mantle cell lymphoma.
McDermott J, Jimeno A. McDermott J, et al. Drugs Today (Barc). 2014 Apr;50(4):291-300. doi: 10.1358/dot.2014.50.4.2133570. Drugs Today (Barc). 2014. PMID: 24918646 Review. - Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma.
Grommes C, Pastore A, Palaskas N, Tang SS, Campos C, Schartz D, Codega P, Nichol D, Clark O, Hsieh WY, Rohle D, Rosenblum M, Viale A, Tabar VS, Brennan CW, Gavrilovic IT, Kaley TJ, Nolan CP, Omuro A, Pentsova E, Thomas AA, Tsyvkin E, Noy A, Palomba ML, Hamlin P, Sauter CS, Moskowitz CH, Wolfe J, Dogan A, Won M, Glass J, Peak S, Lallana EC, Hatzoglou V, Reiner AS, Gutin PH, Huse JT, Panageas KS, Graeber TG, Schultz N, DeAngelis LM, Mellinghoff IK. Grommes C, et al. Cancer Discov. 2017 Sep;7(9):1018-1029. doi: 10.1158/2159-8290.CD-17-0613. Epub 2017 Jun 15. Cancer Discov. 2017. PMID: 28619981 Free PMC article. Clinical Trial. - Current Status of Bruton's Tyrosine Kinase Inhibitor Development and Use in B-Cell Malignancies.
Aw A, Brown JR. Aw A, et al. Drugs Aging. 2017 Jul;34(7):509-527. doi: 10.1007/s40266-017-0468-4. Drugs Aging. 2017. PMID: 28536906 Review.
Cited by
- Comparative Analysis of BTK Inhibitors and Mechanisms Underlying Adverse Effects.
Estupiñán HY, Berglöf A, Zain R, Smith CIE. Estupiñán HY, et al. Front Cell Dev Biol. 2021 Mar 11;9:630942. doi: 10.3389/fcell.2021.630942. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33777941 Free PMC article. Review. - Immunohistochemical analysis indicates that the anatomical location of B-cell non-Hodgkin's lymphoma is determined by differentially expressed chemokine receptors, sphingosine-1-phosphate receptors and integrins.
Middle S, Coupland SE, Taktak A, Kidgell V, Slupsky JR, Pettitt AR, Till KJ. Middle S, et al. Exp Hematol Oncol. 2015 Apr 1;4:10. doi: 10.1186/s40164-015-0004-3. eCollection 2015. Exp Hematol Oncol. 2015. PMID: 25938000 Free PMC article. - Time-Resolved scRNA-Seq Tracks the Adaptation of a Sensitive MCL Cell Line to Ibrutinib Treatment.
Fuhr V, Vafadarnejad E, Dietrich O, Arampatzi P, Riedel A, Saliba AE, Rosenwald A, Rauert-Wunderlich H. Fuhr V, et al. Int J Mol Sci. 2021 Feb 25;22(5):2276. doi: 10.3390/ijms22052276. Int J Mol Sci. 2021. PMID: 33668876 Free PMC article. - The Evolving Role of Bruton's Tyrosine Kinase Inhibitors in B Cell Lymphomas.
Mehra S, Nicholls M, Taylor J. Mehra S, et al. Int J Mol Sci. 2024 Jul 9;25(14):7516. doi: 10.3390/ijms25147516. Int J Mol Sci. 2024. PMID: 39062757 Free PMC article. Review. - Therapy of newly diagnosed follicular lymphoma.
Westin JR, Neelapu SS. Westin JR, et al. Front Oncol. 2012 Dec 11;2:188. doi: 10.3389/fonc.2012.00188. eCollection 2012. Front Oncol. 2012. PMID: 23248775 Free PMC article.
References
- Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat Rev Immunol. 2002;2:945–956. - PubMed
- Fuentes-Pananá EM, Bannish G, Monroe JG. Basal B-cell receptor signaling in B lymphocytes: Mechanisms of regulation and role in positive selection, differentiation, and peripheral survival. Immunol Rev. 2004;197:26–40. - PubMed
- Chiorazzi N, Ferrarini M. B cell chronic lymphocytic leukemia: Lessons learned from studies of the B cell antigen receptor. Annu Rev Immunol. 2003;21:841–894. - PubMed
- Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: Revelations from the B-cell receptor. Blood. 2004;103:4389–4395. - PubMed
- Küppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat Rev Cancer. 2005;5:251–262. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous