Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells - PubMed (original) (raw)
Altered hematopoiesis in trisomy 21 as revealed through in vitro differentiation of isogenic human pluripotent cells
Glenn A Maclean et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Trisomy 21 is associated with hematopoietic abnormalities in the fetal liver, a preleukemic condition termed transient myeloproliferative disorder, and increased incidence of acute megakaryoblastic leukemia. Human trisomy 21 pluripotent cells of various origins, human embryonic stem (hES), and induced pluripotent stem (iPS) cells, were differentiated in vitro as a model to recapitulate the effects of trisomy on hematopoiesis. To mitigate clonal variation, we isolated disomic and trisomic subclones from the same parental iPS line, thereby generating subclones isogenic except for chromosome 21. Under differentiation conditions favoring development of fetal liver-like, γ-globin expressing, definitive hematopoiesis, we found that trisomic cells of hES, iPS, or isogenic origins exhibited a two- to fivefold increase in a population of CD43(+)(Leukosialin)/CD235(+)(Glycophorin A) hematopoietic cells, accompanied by increased multilineage colony-forming potential in colony-forming assays. These findings establish an intrinsic disturbance of multilineage myeloid hematopoiesis in trisomy 21 at the fetal liver stage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
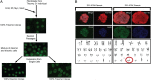
Isolation and characterization of isogenic disomic and trisomic clones. (A) Overview depicting subcloning of disomic and trisomic isogenic iPS cells from a mixed culture of cells. (B) Subcloned isogenic disomic and trisomic clones express Oct4, Tra1-60, and exhibit stable karyotypes lacking chromosomal abnormalities other than trisomy 21. (Magnification: 20×.)
Fig. 2.
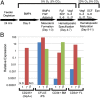
Fetal-like definitive hematopoietic differentiation via EBs. (A) Schematic detailing the differentiation of human pluripotent cells into hematopoietic lineages via EBs. (B) Globin expression in cells isolated from day 10 EBs and CFU-E colonies relative to GAPDH. CD34+ bone marrow and CD34+ FL represent positive control samples from differentiated CD34+ bone marrow and FL cells, respectively. N.D., not detected.
Fig. 3.
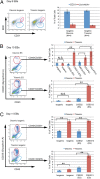
Hematopoietic populations of in vitro differentiated disomic and trisomic pluripotent cells. (A) CD31/KDR+ cells derived from day 8 EBs. (B and C) CD43 and CD235+ populations isolated from day 10 and day 11 EBs, respectively. Error bars represent SEM, and P values were determined by the Student t test; **P < 0.005.
Fig. 4.
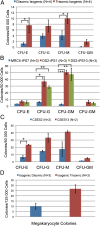
Colony-forming potential of differentiated pluripotent cells. Colonies formed by isogenic (A), ES (B), and independent iPS (C) in methylcellulose colony-forming assay. (D) Megakaryocyte colonies formed by isogenic cells. Error bars represent SEM, and P values were determined by the Student t test; *P < 0.05, **P < 0.005.
Comment in
- Leukaemia: Early changes.
McCarthy N. McCarthy N. Nat Rev Cancer. 2012 Dec;12(12):799. doi: 10.1038/nrc3403. Epub 2012 Nov 15. Nat Rev Cancer. 2012. PMID: 23151601 No abstract available.
Similar articles
- Trisomy 21-associated defects in human primitive hematopoiesis revealed through induced pluripotent stem cells.
Chou ST, Byrska-Bishop M, Tober JM, Yao Y, Vandorn D, Opalinska JB, Mills JA, Choi JK, Speck NA, Gadue P, Hardison RC, Nemiroff RL, French DL, Weiss MJ. Chou ST, et al. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17573-8. doi: 10.1073/pnas.1211175109. Epub 2012 Oct 8. Proc Natl Acad Sci U S A. 2012. PMID: 23045704 Free PMC article. - Downregulation of Endothelin Receptor B Contributes to Defective B Cell Lymphopoiesis in Trisomy 21 Pluripotent Stem Cells.
MacLean GA, McEldoon J, Huang J, Allred J, Canver MC, Orkin SH. MacLean GA, et al. Sci Rep. 2018 May 22;8(1):8001. doi: 10.1038/s41598-018-26123-y. Sci Rep. 2018. PMID: 29789608 Free PMC article. - Spontaneous reversal of the developmental aging of normal human cells following transcriptional reprogramming.
Vaziri H, Chapman KB, Guigova A, Teichroeb J, Lacher MD, Sternberg H, Singec I, Briggs L, Wheeler J, Sampathkumar J, Gonzalez R, Larocca D, Murai J, Snyder E, Andrews WH, Funk WD, West MD. Vaziri H, et al. Regen Med. 2010 May;5(3):345-63. doi: 10.2217/rme.10.21. Regen Med. 2010. PMID: 20230312 - The impact of trisomy 21 on foetal haematopoiesis.
Roberts I, O'Connor D, Roy A, Cowan G, Vyas P. Roberts I, et al. Blood Cells Mol Dis. 2013 Dec;51(4):277-81. doi: 10.1016/j.bcmd.2013.07.008. Epub 2013 Aug 7. Blood Cells Mol Dis. 2013. PMID: 23932236 Free PMC article. Review. - Hematopoiesis from pluripotent stem cell lines.
Sakamoto H, Tsuji-Tamura K, Ogawa M. Sakamoto H, et al. Int J Hematol. 2010 Apr;91(3):384-91. doi: 10.1007/s12185-010-0519-7. Epub 2010 Feb 20. Int J Hematol. 2010. PMID: 20169427 Review.
Cited by
- Consequences of trisomy 21 for brain development in Down syndrome.
Russo ML, Sousa AMM, Bhattacharyya A. Russo ML, et al. Nat Rev Neurosci. 2024 Nov;25(11):740-755. doi: 10.1038/s41583-024-00866-2. Epub 2024 Oct 8. Nat Rev Neurosci. 2024. PMID: 39379691 Review. - GATA1 in Normal and Pathologic Megakaryopoiesis and Platelet Development.
Takasaki K, Chou ST. Takasaki K, et al. Adv Exp Med Biol. 2024;1459:261-287. doi: 10.1007/978-3-031-62731-6_12. Adv Exp Med Biol. 2024. PMID: 39017848 Review. - A dynamic in vitro model of Down syndrome neurogenesis with trisomy 21 gene dosage correction.
Bansal P, Banda EC, Glatt-Deeley HR, Stoddard CE, Linsley JW, Arora N, Deleschaux C, Ahern DT, Kondaveeti Y, Massey RE, Nicouleau M, Wang S, Sabariego-Navarro M, Dierssen M, Finkbeiner S, Pinter SF. Bansal P, et al. Sci Adv. 2024 Jun 7;10(23):eadj0385. doi: 10.1126/sciadv.adj0385. Epub 2024 Jun 7. Sci Adv. 2024. PMID: 38848354 Free PMC article. - Chromosome Transplantation: Opportunities and Limitations.
La Grua A, Rao I, Susani L, Lucchini F, Raimondi E, Vezzoni P, Paulis M. La Grua A, et al. Cells. 2024 Apr 11;13(8):666. doi: 10.3390/cells13080666. Cells. 2024. PMID: 38667281 Free PMC article. Review. - Trisomy 21-driven metabolite alterations are linked to cellular injuries in Down syndrome.
Liu J, Chen S, Huang G, Wen P, Zhou X, Wu Y. Liu J, et al. Cell Mol Life Sci. 2024 Mar 3;81(1):112. doi: 10.1007/s00018-024-05127-0. Cell Mol Life Sci. 2024. PMID: 38433139 Free PMC article.
References
- Bruwier A, Chantrain CF. Hematological disorders and leukemia in children with Down syndrome. Eur J Pediatr. 2011;171:1301–1307. - PubMed
- Roy A, Roberts I, Norton A, Vyas P. Acute megakaryoblastic leukaemia (AMKL) and transient myeloproliferative disorder (TMD) in Down syndrome: A multi-step model of myeloid leukaemogenesis. Br J Haematol. 2009;147:3–12. - PubMed
- Pine SR, et al. Incidence and clinical implications of GATA1 mutations in newborns with Down syndrome. Blood. 2007;110:2128–2131. - PubMed
- Massey GV, et al. Children’s Oncology Group (COG) A prospective study of the natural history of transient leukemia (TL) in neonates with Down syndrome (DS): Children’s Oncology Group (COG) study POG-9481. Blood. 2006;107:4606–4613. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials