Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy - PubMed (original) (raw)
. 2012 Oct 23;109(43):17561-6.
doi: 10.1073/pnas.1215397109. Epub 2012 Oct 8.
Jianping Yuan, Elda Righi, Walid S Kamoun, Marek Ancukiewicz, Jean Nezivar, Michael Santosuosso, John D Martin, Margaret R Martin, Fabrizio Vianello, Pierre Leblanc, Lance L Munn, Peigen Huang, Dan G Duda, Dai Fukumura, Rakesh K Jain, Mark C Poznansky
Affiliations
- PMID: 23045683
- PMCID: PMC3491458
- DOI: 10.1073/pnas.1215397109
Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy
Yuhui Huang et al. Proc Natl Acad Sci U S A. 2012.
Abstract
The recent approval of a prostate cancer vaccine has renewed hope for anticancer immunotherapies. However, the immunosuppressive tumor microenvironment may limit the effectiveness of current immunotherapies. Antiangiogenic agents have the potential to modulate the tumor microenvironment and improve immunotherapy, but they often are used at high doses in the clinic to prune tumor vessels and paradoxically may compromise various therapies. Here, we demonstrate that targeting tumor vasculature with lower vascular-normalizing doses, but not high antivascular/antiangiogenic doses, of an anti-VEGF receptor 2 (VEGFR2) antibody results in a more homogeneous distribution of functional tumor vessels. Furthermore, lower doses are superior to the high doses in polarizing tumor-associated macrophages from an immune inhibitory M2-like phenotype toward an immune stimulatory M1-like phenotype and in facilitating CD4(+) and CD8(+) T-cell tumor infiltration. Based on this mechanism, scheduling lower-dose anti-VEGFR2 therapy with T-cell activation induced by a whole cancer cell vaccine therapy enhanced anticancer efficacy in a CD8(+) T-cell-dependent manner in both immune-tolerant and immunogenic murine breast cancer models. These findings indicate that vascular-normalizing lower doses of anti-VEGFR2 antibody can reprogram the tumor microenvironment away from immunosuppression toward potentiation of cancer vaccine therapies. Given that the combinations of high doses of bevacizumab with chemotherapy have not improved overall survival of breast cancer patients, our study suggests a strategy to use antiangiogenic agents in breast cancer more effectively with active immunotherapy and potentially other anticancer therapies.
Conflict of interest statement
Conflict of interest statement: R.K.J. received research grants from Dyax, MedImmune, and Roche; consultant fees from Dyax, Enlight, Noxxon, and SynDevRx; owns equity in Enlight, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and Boards of Trustees of H&Q Healthcare Investors and H&Q Life Sciences Investors. M.C.P. serves as a scientific adviser to Evaxion-Biotech and owns equity in Celtaxsys. No reagents or funding from these companies was used in this study. Therefore, there is no significant financial or other competing interest in the work.
Figures
Fig. 1.
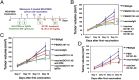
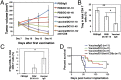
Lower-dose DC101 treatment enhances vaccine therapy in a model of MCaP0008 breast cancer. (A) Treatment protocol. Seven days after implantation of an MCaP0008 breast tumor, mice were divided randomly into two groups and injected i.p. with 5 × 106 CD45−, mitomycin C-treated MCaP0008 tumor tissue cells or with an equal volume of PBS at four time points. These mice subsequently were treated with four doses of DC101 [10 or 40 mg/kg body weight (bw)] at 3-d intervals or with IgG (40 mg/kg bw) 1 d before the fourth vaccination. Mice in the in vivo CD8 depletion study were treated with anti-CD8a or 2A3 (isotype rat IgG2a, 200 μg per mouse) on days −1 (1 d before the first vaccination), 1, 7, and 13. (B) Tumor growth curves. Tumor size was measured every 3 d starting at day 7 after the first vaccination (the first day of DC101 treatment). *P < 0.05, PBS/DC101-10 vs. vaccine/DC101-10. n = 10 mice per group. (C) Depletion of CD8 T-cells abrogated the improvement of quarter-dose DC101 treatment on vaccine therapy. **P < 0.01,vaccine/DC101-10 vs. vaccine/DC101-10/anti-CD8. The IgG2a group had six mice; all other groups had 10 mice. (D) Tumor growth curves. MCaP0008 tumor-bearing mice were treated with rat IgG, half-dose DC101, vaccine, or a combination as described in A. Tumor size was measured at 3-d intervals. *P < 0.05. n = 8–11 mice per group. Data are mean ± SEM.
Fig. 2.
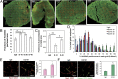
Lower-dose DC101 treatment normalizes breast tumor vasculature. When MCaP0008 tumors reached 4–5 mm in diameter, mice were treated with four doses of DC101 (10, 20, or 40 mg/kg bw) or rat IgG (40 mg/kg bw) as control administered at 3-d intervals. Mice were injected i.v. with 200 μg Hoechst 33342 before tumor harvest on day 11 after DC101 treatment. Perfusion images of whole tumor tissue were taken by multispectral confocal microscopy. (A) Representative perfusion images of whole tumor tissue treated with (Left to Right) IgG, DC101-10, DC101-20, and DC101-40. Green, Sytox staining; red, Hoechst 33342 staining. (Scale bars, 1,000 μm.) (B) The fractions of Hoechst 33342-positive area in whole tumor area. n = 10–14 mice per group. *P < 0.05. (C) The fractions of pimonidazole-positive area in total viable areas. n = 10 mice per group. *P < 0.05, **P < 0.01. (D) A distribution histogram of the Ho33342-positive areas. Tumor areas were subdivided based on a 700-μm grid, the percentage of Hoechst 33342-positive area in each grid was quantified, and their fractions in the total grid were calculated for each tumor. A distribution histogram for each group was plotted. The _x_-axis shows the percentage of Ho33342-positive area in each grid (0.49 mm2). The _y_-axis shows the percentage of Ho33342-positive area in the total grids analyzed. n = 10–14 mice per group. (E) Quantification of pericyte coverage (fraction of area covered) in DC101- and IgG-treated groups (20 mg/kg). CD31-positive endothelial cells are stained red; NG2-positive pericytes are stained green. Confocal images were taken within randomly selected fields excluding the tumor periphery (four to six fields per tumor, six to eight tumors per group). A 20× objective was used for imaging. (Scale bars, 100 μm.) (F) Quantification of tumor vessel perfusion (fraction of area perfused) in DC101- and IgG-treated groups (20 mg/kg). CD31-positive endothelial cells are stained red; FITC-lectin–perfused vessels are stained green. Data are shown as mean ± SEM. **P < 0.01.
Fig. 3.
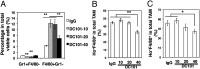
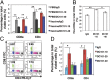
Lower-dose DC101 treatment decreases CD11b+Gr1+ cells, whereas high-dose DC101 treatment decreases the proportion of TAMs proximal to perfused tumor vessels. When tumors reached 4–5 mm in diameter, MCaP0008 or MMTV-PyVT tumor-bearing mice were treated with DC101 or IgG. Tumors were perfused with Hoechst 33342 as described in Fig 2 and then were harvested. (A) Percentages of CD11b+Gr1+ and TAM cells in total viable cells in MCaP0008 tumors. (B) Full-dose DC101 treatment decreased the proportion of Ho+TAMs in total TAMs in MCaP0008 tumors. (C) Full-dose DC101 treatment decreased the proportion of Ho+TAMs in total TAMs in MMTV-PyVT tumors. Data are shown as mean ± SEM. n = 8–10 mice per group in A and B; n = 5 mice per group in C. *P < 0.05, **P < 0.01.
Fig. 4.
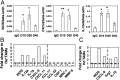
Lower-dose DC101 treatment polarizes perivascular TAMs to an M1-like phenotype. MCaP0008 tumor-bearing mice were treated with DC101 (10, 20, or 40 mg/kg bw) or IgG (40 mg/kg bw) and were perfused with Hoechst33342 as described in Fig. 2. Gene transcription in different TAM populations was analyzed by quantitative real-time PCR (
Table S1
). (A) In MCaP0008 tumors lower-dose (10 and 20 mg/kg bw) DC101 treatments up-regulated typical M1-like gene expression in TAMs as compared with both IgG and full-dose (40 mg/kg bw) DC101 treatment. TAMs were enriched by CD11b-microbead and separated by flow sorting. *P < 0.05, **P < 0.01. (B) Half-dose DC101 treatment elevated expression of M1-like genes and down-regulated expression of M2-like genes in Ho+TAMs. (C) Ho−TAMs displayed a mixed M1/M2-like phenotype after half-dose DC101 treatment. Data are shown as mean ± SEM. TAMs from 8–10 tumors were pooled as three samples in each group. (B and C) Horizontal dash: the value of 1. Vertical dash: separates the genes as M1-type (left side) and M2-type (right side).
Fig. 5.
Lower-dose DC101 treatment promotes the infiltration of T cells into breast cancer parenchyma. MCaP0008 (A and B) and MMTV-PyVT (C and D) tumor-bearing mice were treated with DC101 or rat IgG as described in Figs. 2 and 3. (A) Percentage of CD4+ and CD8+ T cells in total viable cells. n = 10 mice per group. In 7AAD−CD45+ tumor-infiltrating immune cells, lymphoid cells were gated according to the side scatter (SSC) and forward scatter (FSC) and were analyzed for expression of CD4 and CD8a by flow cytometry. *P < 0.05, **P < 0.01. (B) Quarter-dose DC101 treatment increased the proportion of Hoechst 33342-positive CD8+ (Ho+CD8+) T cells in total CD8+ T cells in MCaP0008 tumors. n = 10 mice per group. *P < 0.05, **P < 0.01. (C) Representative flow figures of tumor-infiltrating CD4+ and CD8+ T cells in spontaneous MMTV-PyVT breast tumors. Numbers show the percentages of CD4+ and CD8+ T cells in total viable cells. (D) The percentage of tumor-infiltrating CD4+ and CD8+ T cells in total viable cells. n = 5 mice per group. Data are shown as mean ± SEM. *P < 0.05.
Fig. 6.
Quarter-dose DC101 treatment combined with vaccine is sufficient to maximize anticancer efficacy in the MMTV-PyVT tumor model. When orthotopically transplanted MMTV-PyVT tumors reached 3 mm in diameter, mice received vaccine, DC101, IgG, or anti-CD8 antibody treatments as described in Fig 1_A_. (A) Tumor growth curves. Tumor size was measured at 3-d intervals beginning on day 7 after the first vaccination (the first day of DC101 treatment). The vaccine/DC101-10/anti-CD8 group had 10 mice; all other groups had 11 mice. (B) The percentages of tumor-infiltrating CD4+CD25+Foxp3+ Tregs in the total CD4+ T-cell population. n = 8 mice per group. *P < 0.05, **P < 0.01. (C) Vaccination up-regulated IFN-γ production in MMTV-PyVT tumors. Mitomycin C-treated whole breast tumor tissue cells were used for vaccination. Tumor-infiltrating CD8+ T cells were isolated by anti-CD8 microbeads and then were stimulated by coculturing with mitomycin C-treated whole tumor cells in vitro. IFNγ+CD8+ T cells were analyzed by flow cytometry. n = 8 mice per group. *P < 0.05. (D) The combination of quarter-dose DC101 treatment and vaccine improved survival significantly compared with vaccine monotherapy. Mice were euthanized when tumors reached 1,300 mm3. P < 0.0001, vaccine/DC101-10 vs. vaccine/DC101-10/anti-CD8; P < 0.05, vaccine/IgG vs. vaccine/DC101-10 (log-rank test). The vaccine/DC101-10/anti-CD8 group had seven mice; all other groups had 11 mice. Data are shown as mean ± SEM. *P < 0.05.
Fig. 7.
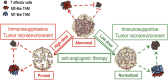
A schematic model showing that lower-dose DC101 treatment reprograms the tumor microenvironment from immunosuppressive to immunosupportive and potentiates cancer vaccine therapy. Abnormal tumor vasculature creates a hypoxic tumor microenvironment, which impedes the infiltration of T effector cells into the tumor and polarizes TAMs to the immune-inhibitory M2-like phenotype that suppresses the function of T effector cells. Lower-dose antiangiogenic treatment normalizes the tumor vasculature and generates a homogeneous distribution of perfused tumor vessels, which promotes the infiltration of T effector cells, redirects TAMs to an immune stimulatory M1-like phenotype, and thereby substantially improves the anticancer efficacy of a cancer vaccine therapy. Conversely, high-dose antiangiogenic treatment prunes tumor vessels, increases hypoxia, fails to induce TAM M1-like polarization, and restricts the infiltration of T effector cells into tumor parenchyma, resulting in impaired cancer vaccine therapy.
Similar articles
- Vascular normalization as an emerging strategy to enhance cancer immunotherapy.
Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Huang Y, et al. Cancer Res. 2013 May 15;73(10):2943-8. doi: 10.1158/0008-5472.CAN-12-4354. Epub 2013 Feb 25. Cancer Res. 2013. PMID: 23440426 Free PMC article. Review. - "γδT Cell-IL17A-Neutrophil" Axis Drives Immunosuppression and Confers Breast Cancer Resistance to High-Dose Anti-VEGFR2 Therapy.
Zhang Z, Yang C, Li L, Zhu Y, Su K, Zhai L, Wang Z, Huang J. Zhang Z, et al. Front Immunol. 2021 Oct 15;12:699478. doi: 10.3389/fimmu.2021.699478. eCollection 2021. Front Immunol. 2021. PMID: 34721375 Free PMC article. - Low-Dose Anti-Angiogenic Therapy Sensitizes Breast Cancer to PD-1 Blockade.
Li Q, Wang Y, Jia W, Deng H, Li G, Deng W, Chen J, Kim BYS, Jiang W, Liu Q, Liu J. Li Q, et al. Clin Cancer Res. 2020 Apr 1;26(7):1712-1724. doi: 10.1158/1078-0432.CCR-19-2179. Epub 2019 Dec 17. Clin Cancer Res. 2020. PMID: 31848190 Clinical Trial. - Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer.
Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Shrimali RK, et al. Cancer Res. 2010 Aug 1;70(15):6171-80. doi: 10.1158/0008-5472.CAN-10-0153. Epub 2010 Jul 14. Cancer Res. 2010. PMID: 20631075 Free PMC article. - [Vascular normalization and cancer immunotherapy].
Zeng J, Yuan D, Liu H, Song Y. Zeng J, et al. Zhongguo Fei Ai Za Zhi. 2014 Mar;17(3):273-6. doi: 10.3779/j.issn.1009-3419.2014.03.16. Zhongguo Fei Ai Za Zhi. 2014. PMID: 24667268 Free PMC article. Review. Chinese.
Cited by
- Hyperbaric Oxygen Boosts PD-1 Antibody Delivery and T Cell Infiltration for Augmented Immune Responses Against Solid Tumors.
Liu X, Ye N, Liu S, Guan J, Deng Q, Zhang Z, Xiao C, Ding ZY, Zhang BX, Chen XP, Li Z, Yang X. Liu X, et al. Adv Sci (Weinh). 2021 Aug;8(15):e2100233. doi: 10.1002/advs.202100233. Epub 2021 Jun 3. Adv Sci (Weinh). 2021. PMID: 34085419 Free PMC article. - Microenvironmental regulation of therapeutic response in cancer.
Klemm F, Joyce JA. Klemm F, et al. Trends Cell Biol. 2015 Apr;25(4):198-213. doi: 10.1016/j.tcb.2014.11.006. Epub 2014 Dec 22. Trends Cell Biol. 2015. PMID: 25540894 Free PMC article. Review. - Dual role of endothelial Myct1 in tumor angiogenesis and tumor immunity.
Kabir AU, Subramanian M, Lee DH, Wang X, Krchma K, Wu J, Naismith T, Halabi CM, Kim JY, Pulous FE, Petrich BG, Kim S, Park HC, Hanson PI, Pan H, Wickline SA, Fremont DH, Park C, Choi K. Kabir AU, et al. Sci Transl Med. 2021 Mar 3;13(583):eabb6731. doi: 10.1126/scitranslmed.abb6731. Sci Transl Med. 2021. PMID: 33658356 Free PMC article. - Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity.
Lee WS, Yang H, Chon HJ, Kim C. Lee WS, et al. Exp Mol Med. 2020 Sep;52(9):1475-1485. doi: 10.1038/s12276-020-00500-y. Epub 2020 Sep 11. Exp Mol Med. 2020. PMID: 32913278 Free PMC article. Review. - Reprogramming the Intrahepatic Cholangiocarcinoma Immune Microenvironment by Chemotherapy and CTLA-4 Blockade Enhances Anti-PD-1 Therapy.
Chen J, Amoozgar Z, Liu X, Aoki S, Liu Z, Shin SM, Matsui A, Hernandez A, Pu Z, Halvorsen S, Lei PJ, Datta M, Zhu L, Ruan Z, Shi L, Staiculescu D, Inoue K, Munn LL, Fukumura D, Huang P, Sassi S, Bardeesy N, Ho WJ, Jain RK, Duda DG. Chen J, et al. Cancer Immunol Res. 2024 Apr 2;12(4):400-412. doi: 10.1158/2326-6066.CIR-23-0486. Cancer Immunol Res. 2024. PMID: 38260999 Free PMC article.
References
- Rosenberg SA, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. - PubMed
- Offringa R. Cancer. Cancer immunotherapy is more than a numbers game. Science. 2006;314:68–69. - PubMed
- Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA126642/CA/NCI NIH HHS/United States
- R01 CA149285/CA/NCI NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- R01-CA096915/CA/NCI NIH HHS/United States
- R01 CA159258/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA096915/CA/NCI NIH HHS/United States
- R01 HL106584/HL/NHLBI NIH HHS/United States
- R01-CA159258/CA/NCI NIH HHS/United States
- R21 CA139168/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R21-CA139168/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials