The social brain in psychiatric and neurological disorders - PubMed (original) (raw)
Review
The social brain in psychiatric and neurological disorders
Daniel P Kennedy et al. Trends Cogn Sci. 2012 Nov.
Abstract
Psychiatric and neurological disorders have historically provided key insights into the structure-function relationships that subserve human social cognition and behavior, informing the concept of the 'social brain'. In this review, we take stock of the current status of this concept, retaining a focus on disorders that impact social behavior. We discuss how the social brain, social cognition, and social behavior are interdependent, and emphasize the important role of development and compensation. We suggest that the social brain, and its dysfunction and recovery, must be understood not in terms of specific structures, but rather in terms of their interaction in large-scale networks.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure 1
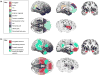
The social brain: from structures to networks. (a) Structures. There are a number of brain regions, only a subset of which are depicted here, that are now known to be involved in social cognition. Some of these are implicated because damage to them impairs aspects of social cognition and behavior; others are implicated because they are differentially activated in healthy brains when people perform social tasks in an MRI scanner. TPJ, temporoparietal junction; dMPFC, dorsomedial prefrontal cortex; STS/STG, superior temporal sulcus/gyrus, FFA: fusiform face area; vMPFC/OFC, ventromedial prefrontal cortex/orbitofrontal cortex. (b) Networks. Several core social cognition networks have been described. Not surprisingly, most of these encompass structures from the original ‘social brain’ [see Panel (a)], although a few new ones have been added, as well. We outline four here. One is a network centered on the amygdala [62,64,135]; the functions of this network (which will likely fractionate into several that are linked to specific amygdala nuclei eventually) range from triggering emotional responses to detecting socially salient stimuli to social affiliative behaviors. A second is the so-called mentalizing network, a collection of structures correlated at rest and activated by thinking about the internal states of others [29,136,137]. A third is recruited when individuals empathize with others [138,139]. A fourth network is activated during observation of the actions of others, including their emotional expressions [28,140,141]. Please note that, for simplicity and clarity, not all regions implicated in the networks are shown; several networks also involve other subcortical and brainstem structures not illustrated here.
Figure 2
Default-mode and social networks from resting-state data. (a) Connectivity of default-mode network regions, many of which are also implicated in social cognition. The central images show lateral and medial views of the brain, with different resting-state networks indicated in different colors (pink is the default-mode). The letters indicate seeds within key nodes of the network, and the surrounding plots show the functional connectivity that seed has with other regions of the brain. Reproduced, with permission, from [113]. (b) Overlap between default-mode network and regions activated by social cognition tasks. Reproduced, with permission, from [115].
Figure 3
Four ‘social’ levels of description and analysis. The levels we discuss in the text suggest particular relationships: the social brain supports social cognition, which then gives rise to social behavior, thus comprising social functioning when integrated over time and context. Although the causal relationships between these levels are complex, this schematic is intended to suggest the concepts associated with each level of description.
Figure 4
Social impairments following bilateral amygdala damage. At the simplest level, the accrued data on the consequences of bilateral amygdala lesions support a dual-process model of sorts. Some of the impairments arise from reduced social attention/perception, likely based on the basolateral amygdale's connections with structures such as basal forebrain and feedback to visual cortices (light blue box on far left: impaired fixations onto and utilization of the eye region in faces). Others arise from reduced emotional response to stimuli, likely based on the central amygdale's connections to hypothalamus, brainstem, and other regions (green box on far right: impaired expression and experience of fear, impaired social distancing). Many social impairments probably arise from both of these mechanisms (dark blue box in middle: anthropomorphizing to shapes, recognizing fear in facial expressions, trustworthiness and approachability judgments from faces). See [62] for a review of these findings.
Figure I
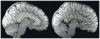
Agenesis of the corpus callosum (AgCC). Saggital structural MRI scans of a typically developed brain (left; outline of the corpus callosum in yellow) and of the brain of a patient with AgCC (right). Images courtesy of Lynn Paul and Mike Tyszka.
Similar articles
- Neural correlates of genetically abnormal social cognition in Williams syndrome.
Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. Meyer-Lindenberg A, et al. Nat Neurosci. 2005 Aug;8(8):991-3. doi: 10.1038/nn1494. Epub 2005 Jul 10. Nat Neurosci. 2005. PMID: 16007084 - Model syndromes for investigating social cognitive and affective neuroscience: a comparison of Autism and Williams syndrome.
Tager-Flusberg H, Skwerer DP, Joseph RM. Tager-Flusberg H, et al. Soc Cogn Affect Neurosci. 2006 Dec;1(3):175-82. doi: 10.1093/scan/nsl035. Soc Cogn Affect Neurosci. 2006. PMID: 18985104 Free PMC article. - Approachability in Williams syndrome.
Jawaid A, Riby DM, Egridere S, Schmolck H, Kass JS, Schulz PE. Jawaid A, et al. Neuropsychologia. 2010 Apr;48(5):1521-3. doi: 10.1016/j.neuropsychologia.2009.12.031. Epub 2009 Dec 24. Neuropsychologia. 2010. PMID: 20035775 No abstract available. - Studies of altered social cognition in neuropsychiatric disorders using functional neuroimaging.
Grady CL, Keightley ML. Grady CL, et al. Can J Psychiatry. 2002 May;47(4):327-36. doi: 10.1177/070674370204700403. Can J Psychiatry. 2002. PMID: 12025431 Review. - [Investigating the "social brain" through Williams syndrome].
Nagamine M, Mimura M, Reiss AL, Hoeft F. Nagamine M, et al. Brain Nerve. 2010 Aug;62(8):877-84. Brain Nerve. 2010. PMID: 20714036 Review. Japanese.
Cited by
- Dysfunction of social cognition and behavior.
Dickerson BC. Dickerson BC. Continuum (Minneap Minn). 2015 Jun;21(3 Behavioral Neurology and Neuropsychiatry):660-77. doi: 10.1212/01.CON.0000466659.05156.1d. Continuum (Minneap Minn). 2015. PMID: 26039847 Free PMC article. Review. - White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration.
Downey LE, Mahoney CJ, Buckley AH, Golden HL, Henley SM, Schmitz N, Schott JM, Simpson IJ, Ourselin S, Fox NC, Crutch SJ, Warren JD. Downey LE, et al. Neuroimage Clin. 2015 Jun 23;8:640-51. doi: 10.1016/j.nicl.2015.06.005. eCollection 2015. Neuroimage Clin. 2015. PMID: 26236629 Free PMC article. - Increased Functional Connectivity Between Subcortical and Cortical Resting-State Networks in Autism Spectrum Disorder.
Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. Cerliani L, et al. JAMA Psychiatry. 2015 Aug;72(8):767-77. doi: 10.1001/jamapsychiatry.2015.0101. JAMA Psychiatry. 2015. PMID: 26061743 Free PMC article. - Towards a comprehensive approach to mentalization-based treatment for children with autism: integrating attachment, neurosciences, and mentalizing.
Costa-Cordella S, Soto-Icaza P, Borgeaud K, Grasso-Cladera A, Malberg NT. Costa-Cordella S, et al. Front Psychiatry. 2023 Nov 30;14:1259432. doi: 10.3389/fpsyt.2023.1259432. eCollection 2023. Front Psychiatry. 2023. PMID: 38098626 Free PMC article. - Compensating for age limits through emotional crossmodal integration.
Chaby L, Boullay VL, Chetouani M, Plaza M. Chaby L, et al. Front Psychol. 2015 May 27;6:691. doi: 10.3389/fpsyg.2015.00691. eCollection 2015. Front Psychol. 2015. PMID: 26074845 Free PMC article.
References
- Klin A, et al. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. - PubMed
- Pelphrey KA, et al. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–261. - PubMed
- Spezio ML, et al. Analysis of face gaze in autism using ‘Bubbles’. Neuropsychologia. 2007;45:144–151. - PubMed
- Damasio AR, et al. Face agnosia and the neural substrates of memory. Annu Rev Neurosci. 1990;13:89–109. - PubMed
Publication types
MeSH terms
Grants and funding
- P50 MH094258/MH/NIMH NIH HHS/United States
- R01 MH080721/MH/NIMH NIH HHS/United States
- K99 MH094409/MH/NIMH NIH HHS/United States
- R01MH080721/MH/NIMH NIH HHS/United States
- P50MH094258/MH/NIMH NIH HHS/United States
- R00 MH094409/MH/NIMH NIH HHS/United States
- K99MH094409/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical