Human nucleoporins promote HIV-1 docking at the nuclear pore, nuclear import and integration - PubMed (original) (raw)
Human nucleoporins promote HIV-1 docking at the nuclear pore, nuclear import and integration
Francesca Di Nunzio et al. PLoS One. 2012.
Erratum in
- PLoS One. 2013;8(12). doi:10.1371/annotation/34f46796-5d53-4a23-b3df-b599b0369eb9
Abstract
The nuclear pore complex (NPC) mediates nucleo-cytoplasmic transport of macromolecules and is an obligatory point of passage and functional bottleneck in the replication of some viruses. The Human Immunodeficiency Virus (HIV) has evolved the required mechanisms for active nuclear import of its genome through the NPC. However the mechanisms by which the NPC allows or even assists HIV translocation are still unknown. We investigated the involvement of four key nucleoporins in HIV-1 docking, translocation, and integration: Nup358/RanBP2, Nup214/CAN, Nup98 and Nup153. Although all induce defects in infectivity when depleted, only Nup153 actually showed any evidence of participating in HIV-1 translocation through the nuclear pore. We show that Nup358/RanBP2 mediates docking of HIV-1 cores on NPC cytoplasmic filaments by interacting with the cores and that the C-terminus of Nup358/RanBP2 comprising a cyclophilin-homology domain contributes to binding. We also show that Nup214/CAN and Nup98 play no role in HIV-1 nuclear import per se: Nup214/CAN plays an indirect role in infectivity read-outs through its effect on mRNA export, while the reduction of expression of Nup98 shows a slight reduction in proviral integration. Our work shows the involvement of nucleoporins in diverse and functionally separable steps of HIV infection and nuclear import.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
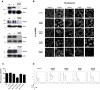
Figure 1. Lentiviral vector-encoded shRNAs achieve efficient knock-down of human nucleoporins and have negligible cytotoxic or cytostatic effects.
Hela cells (4×106) were transduced with lentiviral vectors (MOI 50) encoding shRNAs specific for the indicated nucleoporins and used at 2 days p.t for Nup153 shRNA and 5 days p.t for all others. (A) Knock-down was assessed by Western blotting using specific antibodies against the targeted nucleoporins in non-transduced (WT), LV-transduced (C) and LV-shRNA transduced cells. ß-actin labelling serves as loading control. Results are representative of at least 3 independent experiments. Numbers to the left of Western blots indicate sizes in KDa. (B) Subcellular localisation of nuclear pore components upon nucleoporin knock-down was tested by confocal fluorescence microscopy of LV- (Control) and LV-shRNA transduced cells using specific anti-Nup antibodies. Images were acquired on the same day with the same conditions and are representative of two independent experiments. (C) Cell viability was determined by detecting mitochondrial activity in living cells using the MTT assay. Results show the mean of two experiments carried out in triplicates +/− SD. (D) Cell cycling was assessed by propidium iodide labelling followed by flow cytometry. x and y ordinates show propidium iodide fluorescence and cell counts, respectively.
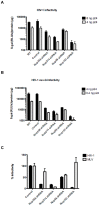
Figure 2. All tested nucleoporins disrupt HIV-1 infectivity when depleted but have only a limited effect on MLV infection.
(A) The effect of nucleoporin knock-down on HIV-1 infectivity was assessed in P4-CCR5 indicator cells using a single cycle infectivity assay based on ß-galactosidase expression following infection with wild-type HIV-1 (8 or 4 ng) or (B) pseudotyped HIV-1 VSV-G (4 or 0.4 ng). Graphs show with log10 scale the mean luminescence values normalised for protein content +/− SEM and are each representative of 5 independent experiments. (C) Nup358/RanBP2, and Nup153 are specific for HIV-1 infection without effect on MLV infection. LV-shRNA transduced and control cells were infected with 5 ng of p24 of VSV-G pseudotyped NL4.3 luc virus and 5 ul of MLV luc/VSVG. Cell lysates were measured for luciferase activity 48 hr p.i. Luciferase values show averages of triplicate infections normalised by µg of proteins +/− SD.
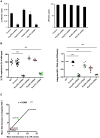
Figure 3. Implication of tested nucleoporins in HIV-1 nuclear import and integration.
(A) P4-CCR5 cells depleted for each of the indicated nucleoporins and control cells were infected with VSV-G pseudotyped NL 4.3 luc virus. 2-LTR circle levels were assessed by quantitative PCR at 24 hr p.i (left panel). As a control for virus input, viral late reverse transcription (LRT) levels were analysed at 7 hr p.i (right panel, log10 scale). Both panels show average results of two independent infection experiments (mean ± SEM), normalised for actin copy number. Infections carried out in the presence of Nevirapine 5 µM led to undetectable levels of both 2-LTR circles and LRT. (B) HIV-1 nuclear import and integration were assessed in parallel in control and knock-down cells at 24 hr p.i. using quantitative PCR of 2-LTR circles and Alu-PCR, respectively. Graphs show individual values from three independent experiments with wild-type HIV-1 or HIV-1 VSV-G virus and the mean +/− SEM. Statistical significance was assessed using one-way Anova with Dunnet post-hoc (*** p<0.0001). (C) Correlation of mean fold decreases in 2-LTR circles and Alu-PCR signals obtained from panel B. Statistical significance was assessed by Pearson coefficient.
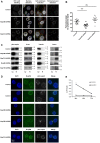
Figure 4. Nup358/RanBP2 mediates HIV-1 docking at the nuclear pore.
(A) Control and Nup214/CAN or Nup358/RanBP2 knock-down cells were infected at 5 days p.t with HIV-1 VSV-G and fixed at 6 h p.i. Cells were labelled with anti-p24 HIV-1 capsid antibody, stained with Hoechst, and observed by confocal fluorescence microscopy. An Acapella script was used to segment the nucleus and cytoplasm of each cell. Nuclear and cell membrane detection were derived from Hoechst staining and LV-encoded GFP expression, respectively. HIV-1 p24 capsid signals were automatically quantified within perinuclear or cytoplasmic regions using a spot detection algorithm, with an intensity threshold set on negative controls. Perinuclear spots, defined as those present within 2 pixels of the nuclear membrane (480 nm), are highlighted as coloured dots in right-hand panels. (B) Quantification of Acapella-detected perinuclear p24 signal. Each point corresponds to one random cell and indicates the number of perinuclear capsid signals as a percentage of total cytoplasmic signal, representative of three independent experiments with mean +/− SEM. (C) Control or LV-shRNA transduced cells were infected with HIV-1 VSV-G and fractionated at 6 hr p.i. At this early time point, reverse transcription is not completed and the majority of viral complexes is still in the cytoplasm: p24 capsid signal in the nuclear fraction corresponds to viruses docked at the nuclear membrane. Nuclear and cytoplasmic fractions were tested for p24 content by Western blotting. Actin, tubulin and lamin labelling were carried out on the same samples to test for protein loading and integrity of cytoplasmic and nuclear fractions, respectively. Numbers indicate sizes in kDa. (D) Control and Nup358/RanBP2 knock-down cells were infected with HIV-1 VSV-G and fractionated at 6 hr p.i. Nuclei were labelled with anti-p24 and anti-Nup214 or -Nup358 antibodies, stained with Hoechst and placed on coverslips for microscopy observation using Apotome structured illumination (Zeiss). (E) 2-LTR time points in control and Nup358/RanBP2 knock-down cells at 24 h, 48 h and 72 h p.i normalised for actin copy number, mean of triplicates +/− SD.
Figure 5. Nup358/RanBP2 interacts with HIV-1 CA-NC.
(A) Protein sequence alignment of cyclophilin-homology domain of Nup358/RanBP2 and the human cyclophilin A, showing 66% identity (amino acids conserved are in red in capital letters and the amino acids divergent are in lowercase in black). (B) Domain structure of the wild-type and truncated Nup358-RanBP2 GFP fusion proteins used for interaction with CA-NC complexes. LRR: leucine-rich region. R1–R4: RanBP homology domains (RBH-1-4). Zn fingers: zinc finger domains. IR: internal repeats. Cyp: cyclophilin-homology domain. BPN, BPM, BPC: N-terminal, middle and C-terminal regions of Nup358/RanBP2 (Joseph and Dasso, 2008). Microscopy image showing location of the GFP-RanBP2 constructs in 293T transfected cells. (C) _In vitro_-assembled CA–NC complexes were mixed with 293T lysates containing WT GFP-RanBP2 or GFP-RanBP2-ΔCyp or Trim5αRH-HA and layered onto 70% sucrose before centrifugation. Immediately before mixing, an aliquot of the cell lysate was removed and blotted with α-GFP or α-HA antibodies to determine the steady-state expression levels of the different transgenic proteins (input). After centrifugation, the pellet was resuspended in SDS sample buffer and analyzed by Western blotting with an anti-GFP antibody (to detect RanBP2) or anti-HA antibody (to detect Trim5αRH) or an anti-p24 antibody (to detect CA-NC). As control of the assay we included TRIM5α-HA.
Figure 6. Overview of the functional interactions between nucleoporins and HIV-1 during infection.
Left: HIV-1 capsid cores dock at the cytoplasmic filaments of NPCs via an interaction with Nup358/RanBP2. Nup214/CAN does not participate in HIV-1 docking or nuclear import. Its identification as a HIV-1 infectivity co-factor in previous studies is linked to its importance in RNA export from the nucleus. Right: upon uncoating, the viral pre-integration complex (PIC) crosses the lumen of the NPC and exits from the nuclear basket assisted by Nup153. Nup98 does not mediate HIV-1 nuclear import but has a moderate effect on HIV-1 integration in host chromatin.
Similar articles
- The nucleoporin Nup358/RanBP2 promotes nuclear import in a cargo- and transport receptor-specific manner.
Wälde S, Thakar K, Hutten S, Spillner C, Nath A, Rothbauer U, Wiemann S, Kehlenbach RH. Wälde S, et al. Traffic. 2012 Feb;13(2):218-33. doi: 10.1111/j.1600-0854.2011.01302.x. Epub 2011 Nov 21. Traffic. 2012. PMID: 21995724 - Specific armadillo repeat sequences facilitate β-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358.
Sharma M, Jamieson C, Johnson M, Molloy MP, Henderson BR. Sharma M, et al. J Biol Chem. 2012 Jan 6;287(2):819-31. doi: 10.1074/jbc.M111.299099. Epub 2011 Nov 21. J Biol Chem. 2012. PMID: 22110128 Free PMC article. Retracted. - Nup214 is required for CRM1-dependent nuclear protein export in vivo.
Hutten S, Kehlenbach RH. Hutten S, et al. Mol Cell Biol. 2006 Sep;26(18):6772-85. doi: 10.1128/MCB.00342-06. Mol Cell Biol. 2006. PMID: 16943420 Free PMC article. - New insights in the role of nucleoporins: a bridge leading to concerted steps from HIV-1 nuclear entry until integration.
Di Nunzio F. Di Nunzio F. Virus Res. 2013 Dec 26;178(2):187-96. doi: 10.1016/j.virusres.2013.09.003. Epub 2013 Sep 16. Virus Res. 2013. PMID: 24051001 Review. - The Part and the Whole: functions of nucleoporins in nucleocytoplasmic transport.
Wälde S, Kehlenbach RH. Wälde S, et al. Trends Cell Biol. 2010 Aug;20(8):461-9. doi: 10.1016/j.tcb.2010.05.001. Epub 2010 Jun 4. Trends Cell Biol. 2010. PMID: 20627572 Review.
Cited by
- How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain.
Klingler J, Anton H, Réal E, Zeiger M, Moog C, Mély Y, Boutant E. Klingler J, et al. Viruses. 2020 Aug 13;12(8):888. doi: 10.3390/v12080888. Viruses. 2020. PMID: 32823718 Free PMC article. Review. - HIV Capsid Inhibitors Beyond PF74.
McArthur C, Gallazzi F, Quinn TP, Singh K. McArthur C, et al. Diseases. 2019 Oct 30;7(4):56. doi: 10.3390/diseases7040056. Diseases. 2019. PMID: 31671622 Free PMC article. Review. - Capsid-host interactions for HIV-1 ingress.
Jang S, Engelman AN. Jang S, et al. Microbiol Mol Biol Rev. 2023 Dec 20;87(4):e0004822. doi: 10.1128/mmbr.00048-22. Epub 2023 Sep 26. Microbiol Mol Biol Rev. 2023. PMID: 37750702 Free PMC article. Review. - HIV Infection: Shaping the Complex, Dynamic, and Interconnected Network of the Cytoskeleton.
Cabrera-Rodríguez R, Pérez-Yanes S, Lorenzo-Sánchez I, Trujillo-González R, Estévez-Herrera J, García-Luis J, Valenzuela-Fernández A. Cabrera-Rodríguez R, et al. Int J Mol Sci. 2023 Aug 23;24(17):13104. doi: 10.3390/ijms241713104. Int J Mol Sci. 2023. PMID: 37685911 Free PMC article. Review. - Importin β1 targeting by hepatitis C virus NS3/4A protein restricts IRF3 and NF-κB signaling of IFNB1 antiviral response.
Gagné B, Tremblay N, Park AY, Baril M, Lamarre D. Gagné B, et al. Traffic. 2017 Jun;18(6):362-377. doi: 10.1111/tra.12480. Epub 2017 May 2. Traffic. 2017. PMID: 28295920 Free PMC article.
References
- Gorlich D, Mattaj IW (1996) Nucleocytoplasmic transport. Science 271: 1513–1518. - PubMed
- Allen TD, Cronshaw JM, Bagley S, Kiseleva E, Goldberg MW (2000) The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J Cell Sci 113 (Pt 10): 1651–1659. - PubMed
- Terry LJ, Shows EB, Wente SR (2007) Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science 318: 1412–1416. - PubMed
- Rabut G, Doye V, Ellenberg J (2004) Mapping the dynamic organization of the nuclear pore complex inside single living cells. Nat Cell Biol 6: 1114–1121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous