CaMKII determines mitochondrial stress responses in heart - PubMed (original) (raw)
. 2012 Nov 8;491(7423):269-73.
doi: 10.1038/nature11444. Epub 2012 Oct 10.
Olha M Koval, Jingdong Li, B Julie He, Chantal Allamargot, Zhan Gao, Elizabeth D Luczak, Duane D Hall, Brian D Fink, Biyi Chen, Jinying Yang, Steven A Moore, Thomas D Scholz, Stefan Strack, Peter J Mohler, William I Sivitz, Long-Sheng Song, Mark E Anderson
Affiliations
- PMID: 23051746
- PMCID: PMC3471377
- DOI: 10.1038/nature11444
CaMKII determines mitochondrial stress responses in heart
Mei-Ling A Joiner et al. Nature. 2012.
Abstract
Myocardial cell death is initiated by excessive mitochondrial Ca(2+) entry causing Ca(2+) overload, mitochondrial permeability transition pore (mPTP) opening and dissipation of the mitochondrial inner membrane potential (ΔΨm). However, the signalling pathways that control mitochondrial Ca(2+) entry through the inner membrane mitochondrial Ca(2+) uniporter (MCU) are not known. The multifunctional Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) is activated in ischaemia reperfusion, myocardial infarction and neurohumoral injury, common causes of myocardial death and heart failure; these findings suggest that CaMKII could couple disease stress to mitochondrial injury. Here we show that CaMKII promotes mPTP opening and myocardial death by increasing MCU current (I(MCU)). Mitochondrial-targeted CaMKII inhibitory protein or cyclosporin A, an mPTP antagonist with clinical efficacy in ischaemia reperfusion injury, equivalently prevent mPTP opening, ΔΨm deterioration and diminish mitochondrial disruption and programmed cell death in response to ischaemia reperfusion injury. Mice with myocardial and mitochondrial-targeted CaMKII inhibition have reduced I(MCU) and are resistant to ischaemia reperfusion injury, myocardial infarction and neurohumoral injury, suggesting that pathological actions of CaMKII are substantially mediated by increasing I(MCU). Our findings identify CaMKII activity as a central mechanism for mitochondrial Ca(2+) entry in myocardial cell death, and indicate that mitochondrial-targeted CaMKII inhibition could prevent or reduce myocardial death and heart failure in response to common experimental forms of pathophysiological stress.
Figures
Figure 1
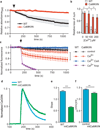
Isolated mitochondria with transgenic, membrane-targeted CaMKII inhibition (CaMKIIN) are resistant to Ca2+ (200 µM Ca2+ free) challenge. a. Mitochondria mPTP Ca2+-dependent (arrow) opening reflected by a decrease in light scattering corresponding to an increase in mitochondrial volume, n = 3/genotype. b. Increasing [Ca2+] promotes loss of ΔΨm more in mitochondria isolated from WT than CaMKIIN mouse hearts (**p < 0.001, ***p < 0.0001, n = 3 hearts/group with duplicate measurements). c. Inner mitochondrial membrane potential (ΔΨm) measurements in isolated mitochondria using a fluorescent reporter, JC-1. Reduced signal from baseline after addition of Ca2+ (200 µM free Ca2+, at arrow) indicates loss of ΔΨm (p < 0.03 for all time points after the Ca2+ challenge) between WT and CaMKIIN treated with Ca2+ alone (indicated with black bracket), red squares versus open red circles, n = 5 hearts/group with duplicate measurements. d. Normalized traces showing rate of mitochondrial Ca2+ uptake in saponin-permeabolized cardiomyocytes after the addition of Ca2+ (arrow, 100 µM free Ca2+). Mitochondrial Ca2+ uptake was monitored in cells by loss of Ca2+ Green-5N fluorescence. The rate of decline in fluorescence reflects the rate of mitochondrial Ca2+ uptake. e. Summary data show the rate of mitochondrial Ca2+ uptake. Nonlinear regression fits for mitochondria Ca2+ uptake between WT and CaMKIIN cardiomyocytes (***p < 0.0001, n = 6/genotype). f. Summary data show reduced Ca2+ uptake in mitochondria, measured using a cameleon mitochondrial-targeted Ca2+ indicator, from HeLa cells expressing mtCaMKIIN compared to myc-expressing controls (**p = 0.003, n = 23 cells/group). Data represent mean ± s.e.m.
Figure 2
CaMKII agonist actions on IMCU require serines 57 and 92. a. IMCU is a Ca2+-dependent conductance. Inset shows IMCU in 0.2, 5 and 100 mM bath [Ca2+] fit with the Hill equation (standard slope). V½ = 23.8 mM, R2= 0.955 and h = 0.057. Red lines show the 95% confidence intervals (runs test p = 0.743). b. Summary data and time course for IMCU recorded with 0.2 mM Ca2+ after obtaining a high resistance seal and mitoplast membrane rupture (time 0) allowing dialysis of CaMKII T/D. Replacing ATP with non-hydrolyzable ATP (non-ATP) analog (both at 0.1 mM) does not allow a CaMKII-dependent increase in IMCU (all mutant CaMKII at 0.5 µM); T/D and ATP; T/D non-ATP; kinase inactive CaMKII (K/M), CaM and ATP, n = 6 (WT), 7 (T/D), 6 (K/M) and 5 (non-ATP), *p < 0.01, **p < 0.001, ***p < 0.0001 at 20 min. c. Summary data for IMCU recorded with 0.2 mM Ca2+ after addition of 100 nM calyculin A (c-A); c-A with T/D CaMKII (0.5 µM), ATP. n = 7 (control), 9 (C-a) and 7 (C-a with T/D), *p < 0.0001. d. Summary data for IMCU from WT, CaMKIIN and mtCaMKIIN mitochondria. Na+ current (150 mM) recorded in the absence of bath (intermembrane space equivalent) Ca2+ or Ru360 (10 nM). **p < 0.001, ***p < 0.0001, n = 7 (cntl groups), 8 (T/D, WT), 9 (T/D, SS/AA) and 6 (Ru360 groups). e. MCU and CaMKII co-immunoprecipitate from mitochondrial lysate. f. Summary data for IMCU recorded from HEK cell mitoplasts with and without transfection of WT or SS/AA MCU mutants. HEK mitoplast Ca+ currents were inhibited by Ru360 (10 nM). For Ca2+ n = 10 (WT and CaMKIIN) and 7 (mtCaMKIIN); Na+ n = 15 (WT), 13 (CaMKIIN) and 12 (mtCaMKIIN) and for Ru360 n = 8 (WT and CaMKIIN), 5 (mtCaMKIIN), ***p < 0.0001. Data represent mean ± s.e.m, except for inset (see a).
Figure 3
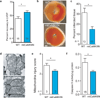
mtCaMKIIN hearts are resistant to I/R injury. a. LVDP recovery following I/R as a percentage of the baseline value (*p = 0.026). b. Representative TTC stained heart sections. The dark red staining represents living myocardium and the solid black outlines form boundaries demarcating viable from dead tissue. c. Summary data from TTC stained hearts showing relative area of infarct normalized to WT, measured as in Supplemental Fig. 2e, *p = 0.006. d. Representative TEM images from hearts treated as in panel a. e. Summary mitochondria injury scores for TEM studies by the criteria used in Supplemental Fig. 2h. (*p = 0.003, more than 500 mitochondria from at least 10 random fields were counted/genotype). f. Caspase 9 activity from hearts treated as in panel a (*p = 0.033, n = number of hearts/genotype). Data represent mean ± s.e.m.
Figure 4
mtCaMKIIN hearts are resistant to apoptosis in vivo. a. Representative images of TUNEL-stained nuclei from WT and mtCaMKIIN heart transverse sections 5 h after MI. Dapi stain shows total number of nuclei. Scale bar indicates 50 µm. b. Upper panel - Summary data for the number of TUNEL-stained nuclei from WT and mtCaMKIIN heart sections 5 h after MI (3 hearts/genotype, 10 images/heart). Lower panel - Summary data for the number of TUNEL-stained nuclei from WT and mtCaMKIIN heart sections 30 min after isoproterenol (ISO) treatment (15 mg/kg, 3 hearts/genotype, 10 images/heart, ***p < 0.0001). Data represent mean ± s.e.m.
Comment in
- CaMKII does it again: even the mitochondria cannot escape its influence.
Correll RN, Molkentin JD. Correll RN, et al. Circ Res. 2013 Apr 26;112(9):1208-11. doi: 10.1161/CIRCRESAHA.113.301263. Circ Res. 2013. PMID: 23620234 No abstract available. - Mitochondrial Ca2+ uniporter and CaMKII in heart.
Fieni F, Johnson DE, Hudmon A, Kirichok Y. Fieni F, et al. Nature. 2014 Sep 25;513(7519):E1-2. doi: 10.1038/nature13626. Nature. 2014. PMID: 25254480 Free PMC article. - Joiner et al. reply.
Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. Joiner ML, et al. Nature. 2014 Sep 25;513(7519):E3. doi: 10.1038/nature13627. Nature. 2014. PMID: 25254481 No abstract available.
Similar articles
- Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate.
Seidlmayer LK, Juettner VV, Kettlewell S, Pavlov EV, Blatter LA, Dedkova EN. Seidlmayer LK, et al. Cardiovasc Res. 2015 May 1;106(2):237-48. doi: 10.1093/cvr/cvv097. Epub 2015 Mar 5. Cardiovasc Res. 2015. PMID: 25742913 Free PMC article. - Cyclophilin D-mediated regulation of the permeability transition pore is altered in mice lacking the mitochondrial calcium uniporter.
Parks RJ, Menazza S, Holmström KM, Amanakis G, Fergusson M, Ma H, Aponte AM, Bernardi P, Finkel T, Murphy E. Parks RJ, et al. Cardiovasc Res. 2019 Feb 1;115(2):385-394. doi: 10.1093/cvr/cvy218. Cardiovasc Res. 2019. PMID: 30165576 Free PMC article. - Serine hydrolase inhibitors block necrotic cell death by preventing calcium overload of the mitochondria and permeability transition pore formation.
Yun B, Lee H, Ghosh M, Cravatt BF, Hsu KL, Bonventre JV, Ewing H, Gelb MH, Leslie CC. Yun B, et al. J Biol Chem. 2014 Jan 17;289(3):1491-504. doi: 10.1074/jbc.M113.497651. Epub 2013 Dec 2. J Biol Chem. 2014. PMID: 24297180 Free PMC article. - The role of mitochondria in protection of the heart by preconditioning.
Halestrap AP, Clarke SJ, Khaliulin I. Halestrap AP, et al. Biochim Biophys Acta. 2007 Aug;1767(8):1007-31. doi: 10.1016/j.bbabio.2007.05.008. Epub 2007 Jun 2. Biochim Biophys Acta. 2007. PMID: 17631856 Free PMC article. Review. - The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury.
Halestrap AP, Richardson AP. Halestrap AP, et al. J Mol Cell Cardiol. 2015 Jan;78:129-41. doi: 10.1016/j.yjmcc.2014.08.018. Epub 2014 Aug 30. J Mol Cell Cardiol. 2015. PMID: 25179911 Review.
Cited by
- Loss of mitochondrial Ca2+ response and CaMKII/ERK activation by LRRK2R1441G mutation correlate with impaired depolarization-induced mitophagy.
Chang EE, Liu H, Choi ZY, Malki Y, Zhang SX, Pang SY, Kung MH, Ramsden DB, Ho SL, Ho PW. Chang EE, et al. Cell Commun Signal. 2024 Oct 10;22(1):485. doi: 10.1186/s12964-024-01844-y. Cell Commun Signal. 2024. PMID: 39390438 Free PMC article. - Structure and function of the mitochondrial calcium uniporter complex.
De Stefani D, Patron M, Rizzuto R. De Stefani D, et al. Biochim Biophys Acta. 2015 Sep;1853(9):2006-11. doi: 10.1016/j.bbamcr.2015.04.008. Epub 2015 Apr 18. Biochim Biophys Acta. 2015. PMID: 25896525 Free PMC article. Review. - Brain activity regulates loose coupling between mitochondrial and cytosolic Ca2+ transients.
Lin Y, Li LL, Nie W, Liu X, Adler A, Xiao C, Lu F, Wang L, Han H, Wang X, Gan WB, Cheng H. Lin Y, et al. Nat Commun. 2019 Nov 21;10(1):5277. doi: 10.1038/s41467-019-13142-0. Nat Commun. 2019. PMID: 31754099 Free PMC article. - Neuronal Epac1 mediates retinal neurodegeneration in mouse models of ocular hypertension.
Liu W, Ha Y, Xia F, Zhu S, Li Y, Shi S, Mei FC, Merkley K, Vizzeri G, Motamedi M, Cheng X, Liu H, Zhang W. Liu W, et al. J Exp Med. 2020 Apr 6;217(4):e20190930. doi: 10.1084/jem.20190930. J Exp Med. 2020. PMID: 31918438 Free PMC article.
References
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. - PubMed
- Clapham DE. Calcium Signaling. Cell. 2007;131:1047–1058. - PubMed
- Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL062494/HL/NHLBI NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- R01 HL113001/HL/NHLBI NIH HHS/United States
- R01 HL62494/HL/NHLBI NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
- R01 HL090905/HL/NHLBI NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- R01 HL70250/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous