The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils - PubMed (original) (raw)
. 2012 Dec 7;279(1748):4724-33.
doi: 10.1098/rspb.2012.1745. Epub 2012 Oct 10.
Matthew Collins, David Harker, James Haile, Charlotte L Oskam, Marie L Hale, Paula F Campos, Jose A Samaniego, M Thomas P Gilbert, Eske Willerslev, Guojie Zhang, R Paul Scofield, Richard N Holdaway, Michael Bunce
Affiliations
- PMID: 23055061
- PMCID: PMC3497090
- DOI: 10.1098/rspb.2012.1745
The half-life of DNA in bone: measuring decay kinetics in 158 dated fossils
Morten E Allentoft et al. Proc Biol Sci. 2012.
Abstract
Claims of extreme survival of DNA have emphasized the need for reliable models of DNA degradation through time. By analysing mitochondrial DNA (mtDNA) from 158 radiocarbon-dated bones of the extinct New Zealand moa, we confirm empirically a long-hypothesized exponential decay relationship. The average DNA half-life within this geographically constrained fossil assemblage was estimated to be 521 years for a 242 bp mtDNA sequence, corresponding to a per nucleotide fragmentation rate (k) of 5.50 × 10(-6) per year. With an effective burial temperature of 13.1°C, the rate is almost 400 times slower than predicted from published kinetic data of in vitro DNA depurination at pH 5. Although best described by an exponential model (R(2) = 0.39), considerable sample-to-sample variance in DNA preservation could not be accounted for by geologic age. This variation likely derives from differences in taphonomy and bone diagenesis, which have confounded previous, less spatially constrained attempts to study DNA decay kinetics. Lastly, by calculating DNA fragmentation rates on Illumina HiSeq data, we show that nuclear DNA has degraded at least twice as fast as mtDNA. These results provide a baseline for predicting long-term DNA survival in bone.
Figures
Figure 1.
DNA fragmentation theory. (a) The exponential relationship caused by random fragmentation of DNA. Post-mortem, the template fragment length (L) distribution follows an exponential decline determined by the proportion of damaged sites (λ). This relationship has been described from both modern and ancient samples [–25]. Here, a fragment size distribution representing λ = 0.02 (2% of the bonds in the DNA backbone are broken). (b) A hypothetical signal of temporal DNA decay, which has, prior to this study, been extremely difficult to demonstrate. The model assumes that the observed damage fraction (λ) can be converted to a rate of decay (k) when the age (T) of a sample is known. It implies that the number of DNA copies of a given length (L) will decline exponentially with time—hence the notion that DNA has a half-life. Here, the theoretical decay kinetics of a 50 bp DNA fragment, assuming a k of 2% per site per year. k is converted to a 50 bp decay rate (_k_50), according to a Poisson distribution as: k_50 = 1 – (e−0.02*_50).
Figure 2.
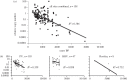
Study site. The three fossil deposits, PV (42°58′22.0″ S, 172°35′49.0″ E), BHV (42°58′19.36″ S, 172°39′56.15″ E) and Rosslea (42°57′53.83″ S, 172°39′22.39″ E), from which 158 radiocarbon-dated moa fossils were characterized for DNA decay kinetics. The sites in North Canterbury, South Island, New Zealand are located within a 5 km radius in the eastern rain shadows of the Southern Alps. Most of the area is more than 200 m a.s.l. and consists of flat alluvial plains and rolling downlands. Information on calibrated radiocarbon ages and DNA preservation (_C_T values) are shown for each site. m, mean age.
Figure 3.
Correlations between age and DNA preservation. Relative mtDNA copy numbers (determined by qPCR) in moa bone plotted against age for all 158 fossils (a), and for each of the three deposits respectively (b). The exponential correlations are significant (p < 0.005) except for the BHV data (p = 0.1) when tested alone. Although a faster average decay is observed at Rosslea, the decay rates (slopes) did not differ significantly from each other.
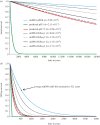
Figure 4.
Observed and predicted rates of DNA decay. The predicted survival of DNA in bone through time, measured as intact phosphodiester bonds in the DNA backbone (a), and survival of a 242 bp fragment (b). The depicted survival rates are based on: (i) the average mtDNA decay rate measured directly from qPCR of 158 moa bones; (ii) the rate of depurination measured from DNA in solution at pH 5 in Lindahl & Nyberg [22] but adjusted to 13.1°C to allow comparison with the moa data; (iii) the same rate adjusted further to pH 7.5, as expected inside a bone; (iv) mtDNA and nuDNA decay rates calculated based on Illumina HiSeq data from two moa samples (HiSeq 1 from sample S40114, HiSeq 2 from sample S39946-3). The estimated decay rate (k, per site per year) is listed for each of the seven datasets.
Similar articles
- Fossil avian eggshell preserves ancient DNA.
Oskam CL, Haile J, McLay E, Rigby P, Allentoft ME, Olsen ME, Bengtsson C, Miller GH, Schwenninger JL, Jacomb C, Walter R, Baynes A, Dortch J, Parker-Pearson M, Gilbert MT, Holdaway RN, Willerslev E, Bunce M. Oskam CL, et al. Proc Biol Sci. 2010 Jul 7;277(1690):1991-2000. doi: 10.1098/rspb.2009.2019. Epub 2010 Mar 10. Proc Biol Sci. 2010. PMID: 20219731 Free PMC article. - Ancient DNA microsatellite analyses of the extinct New Zealand giant moa (Dinornis robustus) identify relatives within a single fossil site.
Allentoft ME, Heller R, Holdaway RN, Bunce M. Allentoft ME, et al. Heredity (Edinb). 2015 Dec;115(6):481-7. doi: 10.1038/hdy.2015.48. Epub 2015 Jun 3. Heredity (Edinb). 2015. PMID: 26039408 Free PMC article. - Rates of evolution in ancient DNA from Adélie penguins.
Lambert DM, Ritchie PA, Millar CD, Holland B, Drummond AJ, Baroni C. Lambert DM, et al. Science. 2002 Mar 22;295(5563):2270-3. doi: 10.1126/science.1068105. Science. 2002. PMID: 11910113 - Moa's Ark or volant ghosts of Gondwana? Insights from nineteen years of ancient DNA research on the extinct moa (Aves: Dinornithiformes) of New Zealand.
Allentoft ME, Rawlence NJ. Allentoft ME, et al. Ann Anat. 2012 Jan 20;194(1):36-51. doi: 10.1016/j.aanat.2011.04.002. Epub 2011 Apr 28. Ann Anat. 2012. PMID: 21596537 Review. - Recent developments in using the molecular decay dating method: a review.
Tintner J. Tintner J. Ann N Y Acad Sci. 2021 Jun;1493(1):29-40. doi: 10.1111/nyas.14560. Epub 2021 Jan 14. Ann N Y Acad Sci. 2021. PMID: 33442875 Free PMC article. Review.
Cited by
- Towards predicting the geographical origin of ancient samples with metagenomic data.
Bozzi D, Neuenschwander S, Cruz Dávalos DI, Sousa da Mota B, Schroeder H, Moreno-Mayar JV, Allentoft ME, Malaspinas AS. Bozzi D, et al. Sci Rep. 2024 Sep 18;14(1):21794. doi: 10.1038/s41598-023-40246-x. Sci Rep. 2024. PMID: 39294129 Free PMC article. - A whole mitochondria analysis of the Tyrolean Iceman's leather provides insights into the animal sources of Copper Age clothing.
O'Sullivan NJ, Teasdale MD, Mattiangeli V, Maixner F, Pinhasi R, Bradley DG, Zink A. O'Sullivan NJ, et al. Sci Rep. 2016 Aug 18;6:31279. doi: 10.1038/srep31279. Sci Rep. 2016. PMID: 27537861 Free PMC article. - AuthentiCT: a model of ancient DNA damage to estimate the proportion of present-day DNA contamination.
Peyrégne S, Peter BM. Peyrégne S, et al. Genome Biol. 2020 Sep 15;21(1):246. doi: 10.1186/s13059-020-02123-y. Genome Biol. 2020. PMID: 32933569 Free PMC article. - Successful extraction of insect DNA from recent copal inclusions: limits and perspectives.
Modi A, Vergata C, Zilli C, Vischioni C, Vai S, Tagliazucchi GM, Lari M, Caramelli D, Taccioli C. Modi A, et al. Sci Rep. 2021 Mar 25;11(1):6851. doi: 10.1038/s41598-021-86058-9. Sci Rep. 2021. PMID: 33767248 Free PMC article. - Design considerations for advancing data storage with synthetic DNA for long-term archiving.
Ezekannagha C, Becker A, Heider D, Hattab G. Ezekannagha C, et al. Mater Today Bio. 2022 May 27;15:100306. doi: 10.1016/j.mtbio.2022.100306. eCollection 2022 Jun. Mater Today Bio. 2022. PMID: 35677811 Free PMC article. Review.
References
- Woodward S. R., Weyand N. J., Bunnell M. 1994. DNA-sequence from Cretaceous period bone fragments. Science 266, 1229–123210.1126/science.7973705 (doi:10.1126/science.7973705) - DOI - DOI - PubMed
- Golenberg E. M., Giannasi D. E., Clegg M. T., Smiley C. J., Durbin M., Henderson D., Zurawski G. 1990. Chloroplast DNA-sequence from a Miocene magnolia species. Nature 344, 656–65810.1038/344656a0 (doi:10.1038/344656a0) - DOI - DOI - PubMed
- Desalle R., Gatesy J., Wheeler W., Grimaldi D. 1992. DNA-sequences from a fossil termite in Oligomiocene amber and their phylogenetic implications. Science 257, 1933–193610.1126/science.1411508 (doi:10.1126/science.1411508) - DOI - DOI - PubMed
- Cano R. J., Poinar H. N., Pieniazek N. J., Acra A., Poinar G. O. 1993. Amplification and sequencing of DNA from a 120–135-million-year-old weevil. Nature 363, 536–53810.1038/363536a0 (doi:10.1038/363536a0) - DOI - DOI - PubMed
- Sutlovic D., Gamulin S., Definis-Gojanovic M., Gugic D., Andjelinovic S. 2008. Interaction of humic acids with human DNA: proposed mechanisms and kinetics. Electrophoresis 29, 1467–147210.1002/Elps.200700699 (doi:10.1002/Elps.200700699) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources