Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody - PubMed (original) (raw)
Enzymatic deglycosylation converts pathogenic neuromyelitis optica anti-aquaporin-4 immunoglobulin G into therapeutic antibody
Lukmanee Tradtrantip et al. Ann Neurol. 2013 Jan.
Abstract
Objective: Neuromyelitis optica (NMO) is caused by binding of pathogenic autoantibodies (NMO-immunoglobulin G [IgG]) to aquaporin-4 (AQP4) on astrocytes, which initiates complement-dependent cytotoxicity (CDC) and inflammation. We recently introduced mutated antibody (aquaporumab) and small-molecule blocker strategies for therapy of NMO, based on prevention of NMO-IgG binding to AQP4. Here, we investigated an alternative strategy involving neutralization of NMO-IgG effector function by selective IgG heavy-chain deglycosylation with bacteria-derived endoglycosidase S (EndoS).
Methods: Cytotoxicity and NMO pathology were measured in cell and spinal cord slice cultures, and in mice exposed to control or EndoS-treated NMO-IgG.
Results: EndoS treatment of NMO patient serum reduced by >95% CDC and antibody-dependent cell-mediated cytotoxicity, without impairment of NMO-IgG binding to AQP4. Cytotoxicity was also prevented by addition of EndoS after NMO-IgG binding to AQP4. The EndoS-treated, nonpathogenic NMO-IgG competitively displaced pathogenic NMO-IgG bound to AQP4, and prevented NMO pathology in spinal cord slice culture and mouse models of NMO.
Interpretation: EndoS deglycosylation converts pathogenic NMO-IgG autoantibodies into therapeutic blocking antibodies. EndoS treatment of blood may be beneficial in NMO, and may be accomplished, for example, by therapeutic apheresis using surface-immobilized EndoS.
Copyright © 2012 American Neurological Association.
Conflict of interest statement
Potential Conflicts of Interest. Drs. Verkman and Tradtrantip are named co-inventors on a patent application filed on EndoS therapy of NMO. The intellectual property is owned by the University of California.
Figures
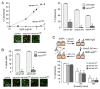
Figure 1. EndoS deglycosylation of NMO-IgG prevents CDC and ADCC
A. (left) Schematic of IgG showing the Fc glycosylation at Asn-297, and Fab binding to AQP4. (right) Sugar moiety at Asn-297 with EndoS cleavage site shown. Asn, asparagine; Fuc, fucose; GlcNac, N-acetylglucosamine; Man, mannose; Gal, galactose; Sial, sialic acid. B. Commassie blue SDS-PAGE and Lens culinaris agglutinin (LCA) lectin blot of control and EndoS-treated NMO-IgG and purified IgG from NMO sera. C. (top) CDC in AQP4-expressing CHO and U87MG cells incubated with NMO-IgG or NMO-IgGGL− and 2% human complement, as quantified by LDH release (S.E., n=4). (bottom) Live/dead (green/red) staining of AQP4-expressing CHO cells incubated with 5 μg/mL NMO-IgG or NMO-IgGGL− and 2% human complement. D. ADCC in AQP4-expressing CHO cells incubated with NK-cells and 20 μg/mL NMO-IgG or NMO-IgGGL−, as quantified by percentage dead cells (S.E., n=4). (bottom) Live/dead (green/red) staining.
Figure 2. EndoS deglycosylation of NMO serum prevents CDC and ADCC
A. (left) CDC in AQP4-expressing CHO cells incubated with NMO serum or NMO serumGL− and 2% human complement, as quantified by LDH release (top) and live/dead staining (bottom). (right) Summary of data from three NMO sera (S.E., n=6, P < 0.001). B. ADCC in AQP4-expressing CHO cells incubated with NK-cells and control or EndoS-treated IgG from NMO sera (1 mg/mL), as quantified by percentage dead cells (S.E., n=5, P < 0.001). (bottom) Live/dead (green/red) staining. C. EndoS addition in situ after NMO-IgG binding to AQP4 reduces CDC. CDC was measured by LDH release in AQP4-expressing CHO cells incubated with NMO serum for 30 min, then treated with EndoS for 30 min, followed by 2% human complement for 1 h. (S.E., n=4, * P < 0.01).
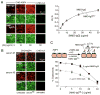
Figure 3. EndoS-treated NMO-IgG binds to AQP4 and competes with binding of pathogenic NMO-IgG
A. Binding of NMO-IgG to AQP4 in CHO cells. (left) Fluorescence micrographs of AQP4-expressing CHO cells stained for NMO-IgG or NMO-IgGGL− (red) and AQP4 (green). (right) Binding of NMO-IgG and NMO-IgGGL− showing red-to-green fluorescence ratio (R/G) as a function of NMO-IgG concentration (S.E., n=3). Differences not significant. B. Binding of control and EndoS-treated NMO patient serum to AQP4 on CHO cells. C. EndoS-treated NMO-IgG protects against CDC caused by (untreated) NMO-IgG. LDH release assayed in CHO cells after 1 h incubation with indicated concentrations of NMO-IgG and NMO-IgGGL−, together with 2% human complement.
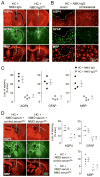
Figure 4. EndoS treatment prevents lesions in an ex vivo spinal cord slice culture model of NMO
A. Spinal cord slice cultures were exposed to 5 μg/mL NMO-IgG or NMO-IgGGL− and 5% human complement (HC). Representative GFAP and AQP4 immunofluorescence shown after 24 h. B. Summary of lesion scores from experiments as in A (S.E., 6 slices per group, * P < 0.01). C. Slice cultures were incubated with 5 μg/mL NMO-IgG, and then 30 min later with 20 U/mL EndoS, and 5 % HC added 60 min later. D. Lesion scores (S.E., 6 slices per group, * P < 0.01).
Figure 5. EndoS treatment prevents lesions in an in vivo mouse model of NMO
A. Brains of live mice were injected with 0.6 μg NMO-IgG or NMO-IgGGL− together with 3 μL human complement (HC). Representative GFAP, AQP4 and myelin (MBP) immunofluorescence at 3 days after injection. Yellow line represents needle tract. White line delimits the lesion with loss of AQP4, GFAP and myelin. B. Higher magnification of brains injected with NMO-IgG and HC. White dashed line delimits the lesion (top). Contralateral hemispheres (non-injected) are shown (right). C. Summary of lesion size from experiments as in A (S.E., 4 mice per group, * P < 0.01 by the non parametric Mann-Whitney test). D. Brains were injected with 30 μg of purified IgG from NMO serum and 105 μg of EndoS-treated IgG purified from the same NMO patient (NMO serumGL−) or a non-NMO control (control serumGL−), together with 3 μL HC. (left) Representative GFAP, AQP4 and MBP immunofluorescence at 3 days after injection. Yellow line shows the needle tract and white line delimits the lesion. (right) Summary lesion size (S.E., 4 mice per group, * P < 0.01 by the non parametric Mann-Whitney test).
Similar articles
- Anti-aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica.
Tradtrantip L, Zhang H, Saadoun S, Phuan PW, Lam C, Papadopoulos MC, Bennett JL, Verkman AS. Tradtrantip L, et al. Ann Neurol. 2012 Mar;71(3):314-22. doi: 10.1002/ana.22657. Epub 2012 Jan 23. Ann Neurol. 2012. PMID: 22271321 Free PMC article. - Therapeutic cleavage of anti-aquaporin-4 autoantibody in neuromyelitis optica by an IgG-selective proteinase.
Tradtrantip L, Asavapanumas N, Verkman AS. Tradtrantip L, et al. Mol Pharmacol. 2013 Jun;83(6):1268-75. doi: 10.1124/mol.113.086470. Epub 2013 Apr 9. Mol Pharmacol. 2013. PMID: 23571414 Free PMC article. - Inhibition of Neuromyelitis Optica Immunoglobulin G Binding to Aquaporin-4 by the Small Molecule Blocker Melanthioidine.
Xu H, Gong Y, Jiao Y, Guo J, Zhao L, Yang J, Tong H, Sun M, Li M. Xu H, et al. Curr Pharm Des. 2023;29(10):793-802. doi: 10.2174/1381612829666230330090953. Curr Pharm Des. 2023. PMID: 36998134 - Neuromyelitis optica: aquaporin-4 based pathogenesis mechanisms and new therapies.
Ratelade J, Verkman AS. Ratelade J, et al. Int J Biochem Cell Biol. 2012 Sep;44(9):1519-30. doi: 10.1016/j.biocel.2012.06.013. Epub 2012 Jun 17. Int J Biochem Cell Biol. 2012. PMID: 22713791 Free PMC article. Review. - Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO.
Verkman AS, Phuan PW, Asavapanumas N, Tradtrantip L. Verkman AS, et al. Brain Pathol. 2013 Nov;23(6):684-95. doi: 10.1111/bpa.12085. Brain Pathol. 2013. PMID: 24118484 Free PMC article. Review.
Cited by
- Ocular adverse events associated with immune checkpoint inhibitors, a scoping review.
Martens A, Schauwvlieghe PP, Madoe A, Casteels I, Aspeslagh S. Martens A, et al. J Ophthalmic Inflamm Infect. 2023 Feb 22;13(1):5. doi: 10.1186/s12348-022-00321-2. J Ophthalmic Inflamm Infect. 2023. PMID: 36811715 Free PMC article. Review. - Treatment strategies for neuromyelitis optica.
Huang TL, Lin KH, Wang JK, Tsai RK. Huang TL, et al. Tzu Chi Med J. 2018 Oct-Dec;30(4):204-208. doi: 10.4103/tcmj.tcmj_102_18. Tzu Chi Med J. 2018. PMID: 30305782 Free PMC article. Review. - ACT001 Relieves NMOSD Symptoms by Reducing Astrocyte Damage with an Autoimmune Antibody.
Li H, Yang M, Song H, Sun M, Zhou H, Fu J, Zhou D, Bai W, Chen B, Lai M, Kang H, Wei S. Li H, et al. Molecules. 2023 Feb 2;28(3):1412. doi: 10.3390/molecules28031412. Molecules. 2023. PMID: 36771078 Free PMC article. - Membrane assembly of aquaporin-4 autoantibodies regulates classical complement activation in neuromyelitis optica.
Soltys J, Liu Y, Ritchie A, Wemlinger S, Schaller K, Schumann H, Owens GP, Bennett JL. Soltys J, et al. J Clin Invest. 2019 Apr 8;129(5):2000-2013. doi: 10.1172/JCI122942. eCollection 2019 Apr 8. J Clin Invest. 2019. PMID: 30958797 Free PMC article. - Optic neuritis in neuromyelitis optica.
Levin MH, Bennett JL, Verkman AS. Levin MH, et al. Prog Retin Eye Res. 2013 Sep;36:159-71. doi: 10.1016/j.preteyeres.2013.03.001. Epub 2013 Mar 30. Prog Retin Eye Res. 2013. PMID: 23545439 Free PMC article.
References
- Jarius S, Paul F, Franciotta D, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008;4:202–214. - PubMed
- Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–815. - PubMed
- Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol. 2010;6:383–392. - PubMed
- Manley GT, Fujimura M, Ma T, et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources