Dab2ip regulates neuronal migration and neurite outgrowth in the developing neocortex - PubMed (original) (raw)
Dab2ip regulates neuronal migration and neurite outgrowth in the developing neocortex
Gum Hwa Lee et al. PLoS One. 2012.
Abstract
Dab2ip (DOC-2/DAB2 interacting protein) is a member of the Ras GTPase-activating protein (GAP) family that has been previously shown to function as a tumor suppressor in several systems. Dab2ip is also highly expressed in the brain where it interacts with Dab1, a key mediator of the Reelin pathway that controls several aspects of brain development and function. We found that Dab2ip is highly expressed in the developing cerebral cortex, but that mutations in the Reelin signaling pathway do not affect its expression. To determine whether Dab2ip plays a role in brain development, we knocked down or over expressed it in neuronal progenitor cells of the embryonic mouse neocortex using in utero electroporation. Dab2ip down-regulation severely disrupts neuronal migration, affecting preferentially late-born principal cortical neurons. Dab2ip overexpression also leads to migration defects. Structure-function experiments in vivo further show that both PH and GRD domains of Dab2ip are important for neuronal migration. A detailed analysis of transfected neurons reveals that Dab2ip down- or up-regulation disrupts the transition from a multipolar to a bipolar neuronal morphology in the intermediate zone. Knock down of Dab2ip in neurons ex-vivo indicates that this protein is necessary for proper neurite development and for the expression of several major neuronal microtubule associated proteins (MAPs), which are important for neurite growth and stabilization. Thus, our study identifies, for the first time, a critical role for Dab2ip in mammalian cortical development and begins to reveal molecular mechanisms that underlie this function.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
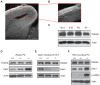
Figure 1. Dab2ip expression in the developing neocortex of wild type and mutant mice.
(A, B) Brain sections obtained from E16.5 mice were immunostained with anti-Dab2ip antibodies. The image in (B) is a magnification of the inset shown in (A). (C) Western blot analysis was performed using neocortex from ICR mice at the indicated embryonic or postnatal ages. The same blot was first probed with Dab2ip antibodies, and then was reprobed with antibodies against actin as an internal loading control. Dab2ip was expressed at all ages throughout the neocortex. The neocortex of newborn wild type (+/+), heterozygous (+/−) and homozygous (−/−) reeler littermates (D), embryonic Dab1 knockout mice (E), and newborn Pten conditional knockout mice (F), was analyzed. Western blots were sequentially probed with Dab2ip, Dab1, phospho- Akt Serine 473 (p-Akt(S)) and actin antibodies. Levels of Dab2ip, unlike Dab1 and pAkt(S), did not differ among genotypes. Scale bars: 100 µm (A), 50 µm (B).
Figure 2. Knock down of Dab2ip by shRNA in transfected and primary cells.
(A) Diagram of Dab2ip, indicating protein functional domains and the targeted regions of Dab2ip shRNAs shDab2ip 1, shDab2ip 2, and shDab2ip 3. PH: Pleckstrin homology, C2: phospholipid/Ca2+ binding motif, GRD: GAP-related domain, NPxY: internalization and Dab1/Dab2 binding motif, PR: proline-rich domain. (B, D) Knock down of exogenous Dab2ip by shDab2ip 1 and shDab2ip 2 in transfected HEK 293T cells. Cells were transfected with a GFP plasmid or co-transfected with a plasmid encoding Dab2ip (isoform L) and a non-targeting shRNA (control) or Dab2ip shRNAs 1 or 2. Both specific shRNAs strongly reduced Dab2ip expression. The plot in (D) shows the results from triplicate experiments. Dab2ip levels were normalized to actin and expressed relative to the control shRNA. Statistical significance was determined by the Student's _t_-test. (C, E) Knock down of endogenous Dab2ip by shDab2ip 1 in primary cortical neurons. Neurons were transfected with non-targeting shRNA (control) or shDab2ip 1, and analyzed at 2 DIV and 4 DIV. The plot in (E) shows the results from triplicate experiments. The data was analyzed as above, except that statistical significance was determined by a one-tailed _t_-test. *, p<0.05; **, p<0.01.
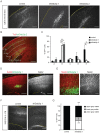
Figure 3. Dab2ip is required for cortical neuron migration.
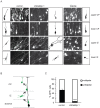
Control shRNA or shDab2ip 1 and 2 were trasnfected into neural progenitor cells of the mouse embryonic neocortex at E14.5 by IUE. The migrating cortical neurons were visualized by co-transfection with a GFP-expressing plasmid. (A) Representative confocal images of the lateral neocortex 4 days after IUE with the indicated shRNA constructs show the distribution of GFP+ cells. The dotted lines indicate the pial surface. Dab2ip knock down neurons failed to migrate, whereas control neurons migrated efficiently toward the surface of the neocortex. (B) GFP autofluorescence (green) and Tuj1 immunofluorescence (red) were used to quantify neuronal migration. Images of the electroporated neocortex were divided into five equal-interval bins. The lowest bin A corresponds to the Tuj1− VZ/SVZ region; bin B corresponds to the IZ and contains many Tuj1+ cells; bins C, D, and E correspond to increasing superficial regions of CP, containing heavily Tuj1+ cells. (C) The percentage of GFP+ neurons in each bin was calculated from multiple electroporated embryos. In the embryos transfected with shDab2ips, most neurons were confined in the IZ (control, 9.5%, n = 3; shDab2ip 1, 64.5%, n = 3; shDab2ip 2, 67.6%, n = 3), and failed to migrate into the CP (control, 49.5%; shDab2ip 1, 6.8%; shDab2ip 2, 3.4%). (D) Sections of brains electroporated with shDab2ip 1 (green) at E14.5 were double-labeled with Satb2 antibodies (red). Arrested knock down neurons in the IZ expressed the Satb2 upper layer marker. (E) Sections adjacent to those imaged in D were double-labeled with Nestin antibodies (red) to label the radial glia scaffold surrounding Dab2ip knock down neurons (green). (F) Representative confocal images of the lateral neocortex of mice transfected at E14.5 and analyzed at postnatal day 8. The neocortical grey matter was divided in two equal bins to quantify the distribution of transfected neurons in upper or lower regions. Many GFP+ neurons transfected with shDab2ip 1, unlike those transfected with control shRNA, remained localized underneath cellular layers (control, 0%, n = 3; shDab2ip 1, 40.8%, n = 3) or in deep layers. CP = cortical plate; IZ = intermediate zone; VZ = ventricular zone; SVZ = subventricular zone. Scale bars: 200 µm (A, B, D, and F), 50 µm (E) *, p<0.05; **, p<0.01.
Figure 4. Dab2ip does not affect to the migration of early-born cortical neurons.
Mouse embryos were electroporated at E12.5 with a GFP-expressing plasmid and control shRNA or shDab2ip 1. Sections of the caudal neocortex were analyzed at E17.5 by GFP autofluorescence (green) and immunofluorescence with Tbr1 antibodies (red). (A) Representative confocal images of the lateral neocortex show that Tbr1+ early-born knock down neurons localize appropriately to the lower CP, whereas Tbr1− late-born neurons fail to migrate to the upper CP. (B) Quantification of the data from multiple experiments. The cortical plate was divided in three bins based on the Tbr1 immunofluorescence signal. Upper CP = region above the Tbr1+ layer; lower CP = region containing most Tbr1+ neurons; under CP = region below the Tbr1+ layer. Scale bars: 100 µm *, p<0.05; **, p<0.01.
Figure 5. Dab2ip overexpression causes migration defects that require intact PH domain and GRD, but not the NPxY motif.
(A) Mouse embryos were electroporated at E14.5 with the indicated Dab2ip expression constructs. Representative confocal images of GFP+ neurons in the developing cortex at E18.5 show that exogenous expression of intact Dab2ip or a construct in which the NPxY motif was mutated cause obvious migration defects. The phenotype was attenuated when constructs carrying a mutation or deletions in the PH domain or GRD were used. (B) Quantification of the data from multiple experiments. The neocortex was divided in two equal bins corresponding to the upper and lower cortex. (C) COS-7 cells and primary cortical neurons were transfected with Myc-tagged Dab2ip of Dab2ipΔPH constructs and processed by immunofluorescence of with anti-Myc antibody. COS-7 cells were analyzed 2 days after transfection and primary cortical neurons were analyzed 3 after transfection. Scale bars: 100 µm (A), 20 µm (C) **, p<0.01; ***, p<0.001.
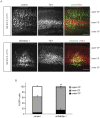
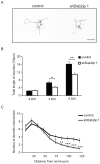
Figure 6. Dab2ip plays a role in the transition from multipolar to bipolar morphology during radial migration.
(A) E14.5 embryos were electroporated in utero with control shRNA, shDab2ip 1 or a Dab2ip expression plasmid, and analyzed at E18.5. High magnification confocal images and tracing of individual, representative GFP+ neurons are shown in different regions of the developing neocortex, as indicated in each row. (B) Diagram of the morphological changes expected in each cortical region during the migration of late-born neurons. Neurons born in the VZ or SVZ move radially into the IZ where they acquire a multipolar morphology. They then convert to a bipolar morphology and undergo glial-guided locomotion to enter the CP. (C) Quantification of the percentage of multipolar neurons in the IZ. The data were obtained from confocal images of multiple sections as in (A). A significantly higher percentage of knock down neurons in the IZ exhibited a multipolar morphology compared to control. Scale bars: 25 µm ***, p<0.001.
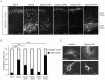
Figure 7. Dab2ip knock down disrupts neurite outgrowth in cultured neurons.
Cortical neurons were isolated 2 days after in utero electroporation and cultured up to 5 days in vitro. (A) Representative images of transfected neurons at 5 DIV using ‘cost’ application in ImageJ. (B) The total length of neurites of Dab2ip knock down neurons and control neurons at 2 DIV, 3 DIV, and 5 DIV was measured using the NeuronJ application in ImageJ. The length of knock down neurites was significantly reduced than control at 3 and 5 DIV. (C) Sholl analysis of transfected neurons at 5 DIV indicates that knock down neurites were significantly shorter than control. Scale bars: 100 µm *, p<0.05; **, p<0.01; ***, p<0.001.
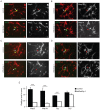
Figure 8. Dab2ip regulates the expression of microtubule-associated proteins.
(A–D) Embryos were electroporated at E14.5 with control shRNA together with GFP and mCherry (double-labeled), or with shDab2ip 1 and GFP alone. Cortical neurons were isolated from transfected embryos at E16.5, cultured for 2 DIV, and subjected to immunofluorescence using Map2 (A), Map1b (B), Tau (C), and Tuj1 (D) specific antibodies, followed by Cy5-labeled secondary antibodies. Confocal images of fields containing neurons positive for GFP/mCherry or GFP alone were acquired. For simplicity, only the GFP and Cy5 channel are shown in the overlay images. Knock down neurons expressed undetectable or low levels of Map2, Map1b and Tau, but normal levels of Tuj1 compared to adjacent or control shRNA-transfected neurons. (E) Quantification of the data collected as in (A–D) from multiple experiments. The fluorescence signal of the soma of transfected neurons was measured relative to untransfected cells in the same field. Ten untransfected cells and 1 transfected cell were analyzed in each field from multiple fields. The data was pooled from >4 independent experiments. Scale bars: 50 µm ***, p<0.001.
Similar articles
- Dab2IP Regulates Neuronal Positioning, Rap1 Activity and Integrin Signaling in the Developing Cortex.
Qiao S, Homayouni R. Qiao S, et al. Dev Neurosci. 2015;37(2):131-41. doi: 10.1159/000369092. Epub 2015 Feb 25. Dev Neurosci. 2015. PMID: 25721469 Free PMC article. - Interaction of Disabled-1 and the GTPase activating protein Dab2IP in mouse brain.
Homayouni R, Magdaleno S, Keshvara L, Rice DS, Curran T. Homayouni R, et al. Brain Res Mol Brain Res. 2003 Jul 23;115(2):121-9. doi: 10.1016/s0169-328x(03)00176-1. Brain Res Mol Brain Res. 2003. PMID: 12877983 - Fyn regulates multipolar-bipolar transition and neurite morphogenesis of migrating neurons in the developing neocortex.
Huang Y, Li G, An L, Fan Y, Cheng X, Li X, Yin Y, Cong R, Chen S, Zhao S. Huang Y, et al. Neuroscience. 2017 Jun 3;352:39-51. doi: 10.1016/j.neuroscience.2017.03.032. Epub 2017 Mar 29. Neuroscience. 2017. PMID: 28363782 - DAB2IP in cancer.
Liu L, Xu C, Hsieh JT, Gong J, Xie D. Liu L, et al. Oncotarget. 2016 Jan 26;7(4):3766-76. doi: 10.18632/oncotarget.6501. Oncotarget. 2016. PMID: 26658103 Free PMC article. Review. - How does Reelin control neuronal migration and layer formation in the developing mammalian neocortex?
Sekine K, Kubo K, Nakajima K. Sekine K, et al. Neurosci Res. 2014 Sep;86:50-8. doi: 10.1016/j.neures.2014.06.004. Epub 2014 Jun 23. Neurosci Res. 2014. PMID: 24969097 Review.
Cited by
- Expression of mouse Dab2ip transcript variants and gene methylation during brain development.
Salami F, Qiao S, Homayouni R. Salami F, et al. Gene. 2015 Aug 15;568(1):19-24. doi: 10.1016/j.gene.2015.05.012. Epub 2015 May 7. Gene. 2015. PMID: 25958345 Free PMC article. - Pumping the brakes on RAS - negative regulators and death effectors of RAS.
Harrell Stewart DR, Clark GJ. Harrell Stewart DR, et al. J Cell Sci. 2020 Feb 10;133(3):jcs238865. doi: 10.1242/jcs.238865. J Cell Sci. 2020. PMID: 32041893 Free PMC article. Review. - Decoding the molecular mechanisms of neuronal migration using in utero electroporation.
Tabata H, Nagata K. Tabata H, et al. Med Mol Morphol. 2016 Jun;49(2):63-75. doi: 10.1007/s00795-015-0127-y. Epub 2015 Nov 25. Med Mol Morphol. 2016. PMID: 26608533 Review. - Urine miRNA signature as potential non-invasive diagnostic biomarker for Hirschsprung's disease.
Sreepada A, Khasanov R, Elkrewi EZ, de la Torre C, Felcht J, Al Abdulqader AA, Martel R, Hoyos-Celis NA, Boettcher M, Wessel LM, Schäfer KH, Tapia-Laliena MÁ. Sreepada A, et al. Front Mol Neurosci. 2025 Jan 8;17:1504424. doi: 10.3389/fnmol.2024.1504424. eCollection 2024. Front Mol Neurosci. 2025. PMID: 39872605 Free PMC article. - New Insights into Reelin-Mediated Signaling Pathways.
Lee GH, D'Arcangelo G. Lee GH, et al. Front Cell Neurosci. 2016 May 9;10:122. doi: 10.3389/fncel.2016.00122. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27242434 Free PMC article. Review.
References
- Rakic P (1972) Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol 145: 61–83. - PubMed
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL (2001) Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci 4: 143–150. - PubMed
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR (2004) Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 7: 136–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous