Neuroinflammatory phenotype in early Alzheimer's disease - PubMed (original) (raw)
Neuroinflammatory phenotype in early Alzheimer's disease
Tiffany L Sudduth et al. Neurobiol Aging. 2013 Apr.
Abstract
Alzheimer's disease (AD) involves progressive neurodegeneration in the presence of misfolded proteins and poorly-understood inflammatory changes. However, research has shown that AD is genetically, clinically, and pathologically heterogeneous. In frozen brain samples of frontal cortex (diseased) and cerebellum (nondiseased) from the University of Kentucky Alzheimer's Disease Center autopsy cohort, we performed gene expression analysis for genes categorizing inflammatory states (termed M1 and M2) from early and late stage AD, and age-matched nondemented controls. We performed analysis of the serum samples for a profile of inflammatory proteins and examined the neuropathologic data on these samples. Striking heterogeneity was found in early AD. Specifically, early-stage AD brain samples indicated apparent polarization toward either the M1 or M2 brain inflammatory states when compared with age-matched nondisease control tissue. This polarization was observed in the frontal cortex and not in cerebellar tissue. We were able to detect differences in AD neuropathology, and changes in serum proteins that distinguished the individuals with apparent M1 versus M2 brain inflammatory polarization.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Figure 1
Early-stage AD brain neuroinflammatory gene expression shows separation into two clusters. Panel A shows the genes that have been measured in the current study. Panel B shows the K-means cluster analysis for the M1 and M2a neuroinflammatory markers that showed significant differences. Panel C shows the cluster means highlighting the pattern of relative gene expression in our two clusters.
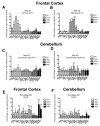
Figure 2
Early-stage AD brain shows a heterogeneous inflammatory response with polarization to the M1 or M2a neuroinflammatory states while the late-stage AD brain does not. Panels A-F show relative gene expression for early-stage AD (A-D) or late-stage AD (E-F) frontal cortex (A-B and E) and cerebellum (C-D and F). The dashed line on each graph represents the mean expression of the age-matched, non-demented controls. * indicates P<0.05, ** indicates P<0.01.
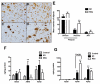
Figure 3
Early-stage AD neuropathology and amyloid load is different between the M1 and M2a neuroinflammatory polarized samples. Panels A-D show Aβ staining in the frontal cortex of M1 polarized samples (A-B) and M2a polarized samples (C-D). Mag = 100X. E shows numbers of diffuse and neuritic plaques and tangles in the frontal cortex. F and G show soluble (F) and insoluble (G) Aβ levels measured by ELISA. ** indicates P<0.01.
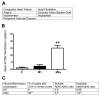
Figure 4
Cerebrovascular disease is more prevalent in the M2a neuroinflammatory polarized samples than the M1 polarized samples. Panel A shows the cerebrovascular risk factors assessed. Panel B shows the mean number of risk factors per patient. Panel G shows CAA prevalence and Aβ40:Aβ42 ratios for each inflammatory group. ** indicates P<0.01.
Figure 5
Several serum proteins can be used to distinguish M1 polarization from M2a polarization. Serum was analyzed for 46 inflammation associated proteins listed in panel A. Panel B shows those proteins that were significantly different between M1 and M2a polarized early-stage AD samples. * indicates P<0.05, ** indicates P<0.01.
Similar articles
- Down syndrome individuals with Alzheimer's disease have a distinct neuroinflammatory phenotype compared to sporadic Alzheimer's disease.
Wilcock DM, Hurban J, Helman AM, Sudduth TL, McCarty KL, Beckett TL, Ferrell JC, Murphy MP, Abner EL, Schmitt FA, Head E. Wilcock DM, et al. Neurobiol Aging. 2015 Sep;36(9):2468-74. doi: 10.1016/j.neurobiolaging.2015.05.016. Epub 2015 May 30. Neurobiol Aging. 2015. PMID: 26103884 Free PMC article. - Neuroinflammatory phenotypes and their roles in Alzheimer's disease.
Wilcock DM. Wilcock DM. Neurodegener Dis. 2014;13(2-3):183-5. doi: 10.1159/000354228. Epub 2013 Sep 6. Neurodegener Dis. 2014. PMID: 24021538 - Neuroinflammation in Alzheimer's disease wanes with age.
Hoozemans JJ, Rozemuller AJ, van Haastert ES, Eikelenboom P, van Gool WA. Hoozemans JJ, et al. J Neuroinflammation. 2011 Dec 7;8:171. doi: 10.1186/1742-2094-8-171. J Neuroinflammation. 2011. PMID: 22152162 Free PMC article. - Microglial polarization: novel therapeutic mechanism against Alzheimer's disease.
Yao K, Zu HB. Yao K, et al. Inflammopharmacology. 2020 Feb;28(1):95-110. doi: 10.1007/s10787-019-00613-5. Epub 2019 Jul 1. Inflammopharmacology. 2020. PMID: 31264132 Review. - Interleukin-18, from neuroinflammation to Alzheimer's disease.
Bossù P, Ciaramella A, Salani F, Vanni D, Palladino I, Caltagirone C, Scapigliati G. Bossù P, et al. Curr Pharm Des. 2010;16(38):4213-24. doi: 10.2174/138161210794519147. Curr Pharm Des. 2010. PMID: 21184660 Review.
Cited by
- Differential compartmentalization of myeloid cell phenotypes and responses towards the CNS in Alzheimer's disease.
Fernández Zapata C, Giacomello G, Spruth EJ, Middeldorp J, Gallaccio G, Dehlinger A, Dames C, Leman JKH, van Dijk RE, Meisel A, Schlickeiser S, Kunkel D, Hol EM, Paul F, Parr MK, Priller J, Böttcher C. Fernández Zapata C, et al. Nat Commun. 2022 Nov 23;13(1):7210. doi: 10.1038/s41467-022-34719-2. Nat Commun. 2022. PMID: 36418303 Free PMC article. - Modeling Alzheimer's disease using human cell derived brain organoids and 3D models.
Fernandes S, Revanna J, Pratt J, Hayes N, Marchetto MC, Gage FH. Fernandes S, et al. Front Neurosci. 2024 Aug 1;18:1434945. doi: 10.3389/fnins.2024.1434945. eCollection 2024. Front Neurosci. 2024. PMID: 39156632 Free PMC article. Review. - Glutamate and GABA in Microglia-Neuron Cross-Talk in Alzheimer's Disease.
Czapski GA, Strosznajder JB. Czapski GA, et al. Int J Mol Sci. 2021 Oct 28;22(21):11677. doi: 10.3390/ijms222111677. Int J Mol Sci. 2021. PMID: 34769106 Free PMC article. Review. - Yizhi Qingxin Formula Extract Ameliorates Cognitive Decline in Aged Rats via the Brain-Derived Neurotrophic Factor/Tropomyosin Receptor Kinase B Pathway.
Ma L, Cao Y, Wang F, Li Z, Wang Z, Yang Y, Pei H, Li H. Ma L, et al. Front Pharmacol. 2020 Apr 30;11:510. doi: 10.3389/fphar.2020.00510. eCollection 2020. Front Pharmacol. 2020. PMID: 32425777 Free PMC article. Retracted. - Sex Differences In Outcomes Of Ablation Of Atrial Fibrillation.
Beck H, Curtis AB. Beck H, et al. J Atr Fibrillation. 2014 Apr 30;6(6):1024. doi: 10.4022/jafib.1024. eCollection 2014 Apr-May. J Atr Fibrillation. 2014. PMID: 27957059 Free PMC article. Review.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiology of aging. 2000;21(3):383–421. - PMC - PubMed
- Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, Ismail MS, Martin BK, Mullan MJ, Sabbagh M, Tariot PN. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(4):402–11. doi:10.1016/j.jalz.2010.12.014. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1RR033173/RR/NCRR NIH HHS/United States
- UL1 RR033173/RR/NCRR NIH HHS/United States
- UL1 TR001998/TR/NCATS NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- UL1 TR000117/TR/NCATS NIH HHS/United States
- P30AG028383/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical