An SMN-dependent U12 splicing event essential for motor circuit function - PubMed (original) (raw)
An SMN-dependent U12 splicing event essential for motor circuit function
Francesco Lotti et al. Cell. 2012.
Abstract
Spinal muscular atrophy (SMA) is a motor neuron disease caused by deficiency of the ubiquitous survival motor neuron (SMN) protein. To define the mechanisms of selective neuronal dysfunction in SMA, we investigated the role of SMN-dependent U12 splicing events in the regulation of motor circuit activity. We show that SMN deficiency perturbs splicing and decreases the expression of a subset of U12 intron-containing genes in mammalian cells and Drosophila larvae. Analysis of these SMN target genes identifies Stasimon as a protein required for motor circuit function. Restoration of Stasimon expression in the motor circuit corrects defects in neuromuscular junction transmission and muscle growth in Drosophila SMN mutants and aberrant motor neuron development in SMN-deficient zebrafish. These findings directly link defective splicing of critical neuronal genes induced by SMN deficiency to motor circuit dysfunction, establishing a molecular framework for the selective pathology of SMA.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
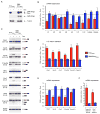
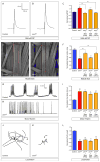
Figure 1. SMN deficiency causes U12 splicing defects in mammalian cells
(A) Western blot analysis of NIH3T3-SmnRNAi and NIH3T3-SMN/SmnRNAi cells cultured without (−) or with (+) Dox for 5 days. (B) RT-qPCR analysis of snRNAs immunoprecipitated with anti-SmB antibodies from NIH3T3-SmnRNAi and NIH3T3-SMN/SmnRNAi cells cultured as in (A). RNA levels in Dox-treated cells were expressed relative to untreated cells (dotted line). (C) RT-PCR analysis of U12 intron-containing genes in NIH3T3-SmnRNAi and NIH3T3-SMN/SmnRNAi cells cultured as in (A). Genes and exons monitored by PCR are indicated on the left. Schematics of spliced and intron-containing mRNAs are shown on the right. Red lines highlight U12 introns. The asterisk indicates a Tmem41b mRNA spliced using donor and acceptor sites located at −30 and +25, respectively, relative to the U12 intron splice sites. (D) RT-qPCR analysis of U12 intron retention for a subset of genes in (C). (E) RT-qPCR analysis of mRNA expression for a subset of genes in (C). (F) RT-qPCR analysis of aberrantly spliced Tmem41b and exon-skipped Clcn7 mRNAs. For all RT-qPCR experiments, NIH3T3 cells were cultured as in (A) and RNA levels in Dox-treated cells were expressed relative to untreated cells (dotted line). Data in all graphs are represented as mean and SEM. See also Figure S1 and Table S1.
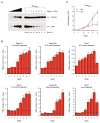
Figure 2. Early onset and time-dependent increase of U12 splicing defects in SMN-deficient mammalian cells
(A) Western blot analysis of NIH3T3-SmnRNAi cells cultured with Dox for the indicated number of days. A two-fold serial dilution of the extract from uninduced cells is shown on the left. (B) RT-qPCR analysis of U12 intron retention in Clcn7, Parp1, Tspan31 and Tmem41b mRNAs as well as accumulation of abnormally spliced Tmem41b and Clcn7 mRNAs in NIH3T3-SmnRNAi cells cultured as in (A). RNA levels in Dox-treated cells were expressed relative to untreated cells (dotted line). (C) SMN deficiency decreases proliferation of NIH3T3 cells. Equal numbers of NIH3T3-SmnRNAi cells were cultured with or without Dox for the indicated number of days and cell number determined at each time point. Data in all graphs are represented as mean and SEM. See also Figure S2.
Figure 3. SMN deficiency decreases snRNA levels and expression of U12 intron-containing genes in Drosophila
(A) Schematic representation of _Drosophila smn_73Ao and _U6atac_K01105 mutants. (B) Western blot analysis of control, _smn_73Ao and _U6atac_K01105 larvae. A two-fold serial dilution of control extract is shown on the left. The asterisk indicates a non-specific protein. (C) Northern blot analysis of snRNA expression in control, _smn_73Ao and _U6atac_K01105 larvae. (D) snRNA levels in _smn_73Ao and _U6atac_K01105 relative to control (dotted line) larvae following normalization to 5.8S rRNA. (E) RT-PCR analysis of U12 intron-containing genes whose expression is affected in both smn73Ao and _U6atac_K01105 compared to control larvae. Genes and exons monitored by PCR are indicated on the left. Schematics of spliced and intron-containing mRNAs are shown on the right. Red lines highlight U12 introns. The –RT lanes correspond to RT-PCR reactions lacking reverse transcriptase. (F) RT-qPCR analysis of U12 intron-containing genes with decreased mRNA expression in both _smn_73Ao and _U6atac_K01105 compared to control larvae. (G) RT-qPCR analysis of genes with increased U12 intron retention in _U6atac_K01105 compared to control larvae. (H) RT-qPCR analysis of genes with increased U12 intron retention in _smn_73Ao compared to control larvae. Data in all graphs are represented as mean and SEM. See also Figure S3 and Table S2.
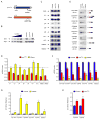
Figure 4. Functional analysis of SMN targets identifies CG8408/Stasimon as a novel gene required for synaptic transmission in Drosophila.
(A) Evoked Excitatory Post-Synaptic Potentials (eEPSPs) in _smn_73A0 and _smn_X7 mutant Drosophila larvae and following mRNA knockdown of the indicated genes by pan-neural expression of UAS-RNAi constructs with C155-Gal4 normalized to control. (B1–B3) Schematic representation of the _stas_EY04008 mutant showing the site of P-element insertion within the 5′ UTR region of the stasimon (CG8408) gene (B1). RT-qPCR analysis of Stasimon mRNA levels in control and stasEY04008 larvae (B2). Normalized eEPSP amplitude in control and stasEY04008 larvae with or without expression of UAS-Stasimon with the pan-neuronal nsyb-Gal4 driver relative to control (B3). (C) Representative eEPSP traces from larvae with Stasimon RNAi in all neurons (C155-Gal4; PAN), motor neurons (OK371-Gal4; MN) or cholinergic neurons (Cha-Gal4; CHOL) normalized to control. (D) Quantification of eEPSP amplitudes in larvae with Stasimon RNAi in specific neuronal types normalized to control. Data in all graphs are represented as mean and SEM. See also Figure S4.
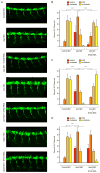
Figure 5. Expression of S_tasimon_ rescues neurotransmitter release at the NMJ and ameliorates muscle size defects in Drosophila SMN mutants
(A–B) Representative eEPSP traces recorded from muscle 6 of segment A3 in control and _smn_X7 larvae. (C) Normalized eEPSP amplitude of _smn_X7 mutants alone or with transgenic UAS-Stasimon expression in all neurons (nsyb-Gal4; PAN), motor neurons (OK371-Gal4; MN) or cholinergic neurons (Cha-Gal4; CHOL) relative to controls. (D–E) Representative images of muscles from segment A3 of control and _smn_X7 larvae labeled with TRITC-phalloidin. (F) Normalized muscle surface area of _smn_X7 mutants alone or expressing Stasimon with the same drivers described in (C) relative to controls. (G–H) Representative recordings of motor rhythms from control and _smn_X7 larvae. (I) Normalized inter-spike intervals of _smn_X7 mutants alone or expressing Stasimon with the same drivers described in (C) relative to controls. (J–K) Representative images of 10 superimposed locomotion path traces from control and _smn_X7 larvae. (L) Normalized path length of _smn_X7 mutants alone or expressing Stasimon with the same drivers described in (C) relative to controls. Data in all graphs are represented as mean and SEM. See also Figure S5.
Figure 6. S_tasimon_ is required for motor neuron development and rescues SMN-dependent motor axon defects in zebrafish embryos
(A) Representative lateral views of motor axons in Tg(mnx1:GFP) zebrafish embryos expressing GFP in motor neurons and injected with Control MO as well as stas MO, smn MO and tdp43 MO either with or without co-injected human STAS RNA. (B) Quantification of the effects of Stasimon deficiency on motor axon development in zebrafish. Motor axons were scored in Tg(mnx1:GFP) embryos injected with Control MO, stas MO or stas MO + STAS RNA. Embryos were classified as severe, moderate, mild or no defects based on the severity of motor axons defects and the percentage for each group is shown. (C) Quantification of Stasimon effects on SMN-dependent motor axons defects in zebrafish. Motor axons were scored in Tg(mnx1:GFP) embryos injected with Control MO, smn MO or smn MO + STAS RNA and embryos were classified as in (B). (D) Quantification of Stasimon effects on TDP43-dependent motor axons defects in zebrafish. Motor axons were scored in Tg(mnx1:GFP) embryos injected with Control MO, tdp43 MO or tdp43 MO + STAS RNA and embryos were classified as in (B). Data in all graphs are represented as mean and SEM. See also Figure S6.
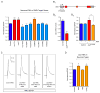
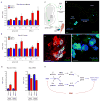
Figure 7. SMN deficiency disrupts Stasimon U12 splicing and mRNA expression in the sensory-motor circuit of SMA mice
(A) RT-qPCR analysis of aberrantly spliced Stasimon mRNA in the spinal cord and L1 DRG from control and SMA mice at the indicated post-natal days. (B). RT-qPCR analysis of Stasimon U12 intron retention in the spinal cord and L1 DRG from control and SMA mice at the indicated post-natal days. (C) Strategy for labeling motor neurons and proprioceptive neurons of the motor circuit by CTb-488 injection in the iliopsoas muscle. (D) Confocal image of CTb-488-labelled iliopsoas motor neurons and DRG neurons from a control mouse. Scale bar, 100 μm. (E) Confocal image showing co-localization of CTb-488 (green) and ChAT (red) in motor neurons from the ventral horn of a CTb-injected mouse. Scale bar, 50 μm. (F) Confocal image showing co-localization of CTb-488 (green) and parvalbumin (blue) in proprioceptive neurons from the DRG of a CTb-injected mouse. Scale bar, 20 μm. (G) RT-qPCR analysis of Stasimon U12 intron retention and mRNA levels in motor neurons and proprioceptive neurons isolated by LCM from CTb-injected control and SMA mice at P6. (H) Model for the sequence of SMN-dependent molecular events necessary for normal motor circuit function (blue) which are disrupted in SMA (red). Data in all graphs are represented as mean and SEM. See also Figure S7.
Comment in
- A circuit mechanism for neurodegeneration.
Roselli F, Caroni P. Roselli F, et al. Cell. 2012 Oct 12;151(2):250-2. doi: 10.1016/j.cell.2012.09.030. Cell. 2012. PMID: 23063119
Similar articles
- Minor snRNA gene delivery improves the loss of proprioceptive synapses on SMA motor neurons.
Osman EY, Van Alstyne M, Yen PF, Lotti F, Feng Z, Ling KK, Ko CP, Pellizzoni L, Lorson CL. Osman EY, et al. JCI Insight. 2020 Jun 18;5(12):e130574. doi: 10.1172/jci.insight.130574. JCI Insight. 2020. PMID: 32516136 Free PMC article. - Developmental arrest of Drosophila survival motor neuron (Smn) mutants accounts for differences in expression of minor intron-containing genes.
Garcia EL, Lu Z, Meers MP, Praveen K, Matera AG. Garcia EL, et al. RNA. 2013 Nov;19(11):1510-6. doi: 10.1261/rna.038919.113. Epub 2013 Sep 4. RNA. 2013. PMID: 24006466 Free PMC article. - SMN is required for sensory-motor circuit function in Drosophila.
Imlach WL, Beck ES, Choi BJ, Lotti F, Pellizzoni L, McCabe BD. Imlach WL, et al. Cell. 2012 Oct 12;151(2):427-39. doi: 10.1016/j.cell.2012.09.011. Cell. 2012. PMID: 23063130 Free PMC article. - Spinal muscular atrophy: Selective motor neuron loss and global defect in the assembly of ribonucleoproteins.
Beattie CE, Kolb SJ. Beattie CE, et al. Brain Res. 2018 Aug 15;1693(Pt A):92-97. doi: 10.1016/j.brainres.2018.02.022. Epub 2018 Feb 17. Brain Res. 2018. PMID: 29462610 Review. - Fishing for a mechanism: using zebrafish to understand spinal muscular atrophy.
Beattie CE, Carrel TL, McWhorter ML. Beattie CE, et al. J Child Neurol. 2007 Aug;22(8):995-1003. doi: 10.1177/0883073807305671. J Child Neurol. 2007. PMID: 17761655 Review.
Cited by
- Behavioral and electrophysiological outcomes of tissue-specific Smn knockdown in Drosophila melanogaster.
Timmerman C, Sanyal S. Timmerman C, et al. Brain Res. 2012 Dec 13;1489:66-80. doi: 10.1016/j.brainres.2012.10.035. Epub 2012 Oct 26. Brain Res. 2012. PMID: 23103409 Free PMC article. - Disease mechanisms and therapeutic approaches in spinal muscular atrophy.
Tisdale S, Pellizzoni L. Tisdale S, et al. J Neurosci. 2015 Jun 10;35(23):8691-700. doi: 10.1523/JNEUROSCI.0417-15.2015. J Neurosci. 2015. PMID: 26063904 Free PMC article. Review. - Circular RNAs as Potential Blood Biomarkers in Amyotrophic Lateral Sclerosis.
Dolinar A, Koritnik B, Glavač D, Ravnik-Glavač M. Dolinar A, et al. Mol Neurobiol. 2019 Dec;56(12):8052-8062. doi: 10.1007/s12035-019-1627-x. Epub 2019 Jun 7. Mol Neurobiol. 2019. PMID: 31175544 Free PMC article. - Splicing efficiency of minor introns in a mouse model of SMA predominantly depends on their branchpoint sequence and can involve the contribution of major spliceosome components.
Jacquier V, Prévot M, Gostan T, Bordonné R, Benkhelifa-Ziyyat S, Barkats M, Soret J. Jacquier V, et al. RNA. 2022 Mar;28(3):303-319. doi: 10.1261/rna.078329.120. Epub 2021 Dec 10. RNA. 2022. PMID: 34893560 Free PMC article. - Molecular Mechanisms Underlying Sensory-Motor Circuit Dysfunction in SMA.
Shorrock HK, Gillingwater TH, Groen EJN. Shorrock HK, et al. Front Mol Neurosci. 2019 Mar 4;12:59. doi: 10.3389/fnmol.2019.00059. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30886572 Free PMC article.
References
- Beattie CE, Carrel TL, McWhorter ML. Fishing for a mechanism: using zebrafish to understand spinal muscular atrophy. J Child Neurol. 2007;22:995–1003. - PubMed
- Boulisfane N, Choleza M, Rage F, Neel H, Soret J, Bordonne R. Impaired minor tri-snRNP assembly generates differential splicing defects of U12-type introns in lymphoblasts derived from a type I SMA patient. Hum Mol Genet. 2011;20:641–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS050414/NS/NINDS NIH HHS/United States
- R01NS069601/NS/NINDS NIH HHS/United States
- T32 GM007367/GM/NIGMS NIH HHS/United States
- R01NS078375/NS/NINDS NIH HHS/United States
- R01 NS078375/NS/NINDS NIH HHS/United States
- R01 NS050414/NS/NINDS NIH HHS/United States
- R21 NS077038/NS/NINDS NIH HHS/United States
- R21NS077038/NS/NINDS NIH HHS/United States
- R01 NS069601/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials