Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation - PubMed (original) (raw)
Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation
Elizabeth A Townsend et al. Am J Respir Cell Mol Biol. 2013 Feb.
Abstract
The prevalence of asthma has increased in recent years, and is characterized by airway hyperresponsiveness and inflammation. Many patients report using alternative therapies to self-treat asthma symptoms as adjuncts to short-acting and long-acting β-agonists and inhaled corticosteroids (ICS). As many as 40% of patients with asthma use herbal therapies to manage asthma symptoms, often without proven efficacy or known mechanisms of action. Therefore, investigations of both the therapeutic and possible detrimental effects of isolated components of herbal treatments on the airway are important. We hypothesized that ginger and its active components induce bronchodilation by modulating intracellular calcium ([Ca(2+)](i)) in airway smooth muscle (ASM). In isolated human ASM, ginger caused significant and rapid relaxation. Four purified constituents of ginger were subsequently tested for ASM relaxant properties in both guinea pig and human tracheas: [6]-gingerol, [8]-gingerol, and [6]-shogaol induced rapid relaxation of precontracted ASM (100-300 μM), whereas [10]-gingerol failed to induce relaxation. In human ASM cells, exposure to [6]-gingerol, [8]-gingerol, and [6]-shogaol, but not [10]-gingerol (100 μM), blunted subsequent Ca(2+) responses to bradykinin (10 μM) and S-(-)-Bay K 8644 (10 μM). In A/J mice, the nebulization of [8]-gingerol (100 μM), 15 minutes before methacholine challenge, significantly attenuated airway resistance, compared with vehicle. Taken together, these novel data show that ginger and its isolated active components, [6]-gingerol, [8]-gingerol, and [6]-shogaol, relax ASM, and [8]-gingerol attenuates airway hyperresponsiveness, in part by altering [Ca(2+)](i) regulation. These purified compounds may provide a therapeutic option alone or in combination with accepted therapeutics, including β(2)-agonists, in airway diseases such as asthma.
Figures
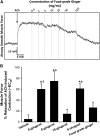
Figure 1.
Ginger and its active constituents relax precontracted human airway smooth muscle. (A) Force studies in human airway smooth muscle show that food-grade ginger relaxes precontracted airways in a dose-dependent manner. Human tracheal strips were contracted to acetylcholine (ACh) at approximately half maximal concentration (∼ EC50), and showed rapid and substantial relaxation upon increasing concentrations of food-grade ginger. In subsequent studies, only the 50 mg/ml concentration was used. (B) Food-grade ginger (50 mg/ml) induced approximately 25% relaxation. The purified active components of crude ginger, [6]-gingerol, [8]-gingerol, and [6]-shogaol, produced relaxation of 60–90% (100 μM). Interestingly, [10]-gingerol (100 μM) did not significantly induce relaxation compared with vehicle control (DMSO, 0.2%). *P < 0.05, compared with vehicle. $P < 0.05, compared with food-grade ginger (n = 5–9).
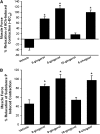
Figure 2.
The relaxant effects of gingerols and [6]-shogaol persist, irrespective of a specific contractile agonist. (A) [6]-gingerol, [8]-gingerol, and [6]-shogaol (300 μM) significantly relaxed both acetylcholine-induced (∼ EC50) and (B) substance P–induced (1 μM) tracheal contractions in ex vivo guinea pig airway smooth muscle. Again, [10]-gingerol did not induce significant relaxation. (A) *P < 0.001, compared with vehicle (n = 8–11). (B) *P < 0.01, compared with vehicle (n = 5–8).
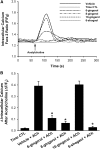
Figure 3.
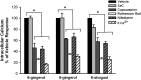
Ginger constituents block acetylcholine-induced increases of intracellular calcium ([Ca2+]i) in human airway smooth muscle cells. (A and B) In Fura-2–loaded human airway smooth muscle cells, pretreatment with 100 μM gingerols or [6]-shogaol, followed by subsequent exposure to 10 μM acetylcholine (ACh), revealed that [6]-gingerol, [8]-gingerol, and [6]-shogaol prevented increases in [Ca2+]i. This result was not observed with [10]-gingerol. (A) Representative tracing. (B) Summary bar graph. *P < 0.05, compared with vehicle (0.2% DMSO; n = 8). s, seconds. CTL, control; F/F0, change in fura-2 fluorescence ratio.
Figure 4.
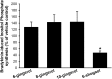
Ginger constituents cause a transient rise in [Ca2+]i. In Fura-2–loaded cells, the addition of [6]-gingerol, [8]-gingerol, or [6]-shogaol alone (100 μM) caused an initial transient increase in [Ca2+]i. This response returned to baseline within 5 minutes. No change in [Ca2+]i was observed with [10]-gingerol (100 μM). *P < 0.05, compared with vehicle (n = 9).
Figure 5.
Initial transient increases in [Ca2+]i caused by ginger components are attributable to influx and store depletion. In airway smooth muscle cells, the initial [Ca2+]i spike was attenuated by the L-type Ca2+ channel blocker, nifedipine (10 μM), the transient receptor potential vanilloid channel blocker, capsazepine (100 μM), the ryanodine receptor antagonist, ruthenium red (20 μM), and the removal of external Ca2+. It was not attenuated by the inositol triphosphate receptor antagonist, xestospongin C (XeC, 1 μM). *P < 0.05 (n = 7–8).
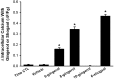
Figure 6.
[6]-shogaol decreases the synthesis of inositol phosphate. After a 15-minute preincubation with ginger constituents (100 μM) or vehicle (0.1% or 0.2% DMSO), 10 μM of bradykinin were added for an additional 30 minutes before the extraction and column separation of newly synthesized total [3H]-inositol phosphates. Only [6]-shogaol significantly attenuated the production of inositol phosphate. Data are expressed as a percentage of vehicle control values. *P < 0.05, compared with vehicle (n = 3–8).
Figure 7.
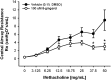
The nebulization of [8]-gingerol prevents hyperresponsiveness to methacholine. In male A/J mice, increasing concentrations of methacholine (MCh) result in increases in central airway resistance (Newtonian resistance, Rn) measured using a constant phase model on a Flexivent FX1 module. Increases in resistance at 37.5 and 50 mg/ml MCh were attenuated in animals that received a 10-second nebulization of 100 μM [8]-gingerol, 15 minutes before MCh challenge. *P < 0.05, compared with vehicle (n = 6–11).
Similar articles
- Active components of ginger potentiate β-agonist-induced relaxation of airway smooth muscle by modulating cytoskeletal regulatory proteins.
Townsend EA, Zhang Y, Xu C, Wakita R, Emala CW. Townsend EA, et al. Am J Respir Cell Mol Biol. 2014 Jan;50(1):115-24. doi: 10.1165/rcmb.2013-0133OC. Am J Respir Cell Mol Biol. 2014. PMID: 23962082 Free PMC article. - Ginger metabolites and metabolite-inspired synthetic products modulate intracellular calcium and relax airway smooth muscle.
Luković E, Perez-Zoghbi JF, Zhang Y, Zhu Y, Sang S, Emala CW. Luković E, et al. Am J Physiol Lung Cell Mol Physiol. 2021 Nov 1;321(5):L912-L924. doi: 10.1152/ajplung.00271.2021. Epub 2021 Sep 22. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34549600 Free PMC article. - Effects of ginger constituents on the gastrointestinal tract: role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors.
Pertz HH, Lehmann J, Roth-Ehrang R, Elz S. Pertz HH, et al. Planta Med. 2011 Jul;77(10):973-8. doi: 10.1055/s-0030-1270747. Epub 2011 Feb 8. Planta Med. 2011. PMID: 21305447 - Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review.
de Lima RMT, Dos Reis AC, de Menezes APM, Santos JVO, Filho JWGO, Ferreira JRO, de Alencar MVOB, da Mata AMOF, Khan IN, Islam A, Uddin SJ, Ali ES, Islam MT, Tripathi S, Mishra SK, Mubarak MS, Melo-Cavalcante AAC. de Lima RMT, et al. Phytother Res. 2018 Oct;32(10):1885-1907. doi: 10.1002/ptr.6134. Epub 2018 Jul 16. Phytother Res. 2018. PMID: 30009484 Review. - Occurrence, biological activity and metabolism of 6-shogaol.
Kou X, Wang X, Ji R, Liu L, Qiao Y, Lou Z, Ma C, Li S, Wang H, Ho CT. Kou X, et al. Food Funct. 2018 Mar 1;9(3):1310-1327. doi: 10.1039/c7fo01354j. Epub 2018 Feb 8. Food Funct. 2018. PMID: 29417118 Review.
Cited by
- Survey on the Traditional Use of Medicinal Herbs in Haiti: A Study on Knowledge, Practices, and Efficacy Prevention.
Thesnor V, Cheremond Y, Sylvestre M, Meffre P, Cebrián-Torrejón G, Benfodda Z. Thesnor V, et al. Plants (Basel). 2024 Aug 26;13(17):2383. doi: 10.3390/plants13172383. Plants (Basel). 2024. PMID: 39273867 Free PMC article. - An Updated Comprehensive Review of Plants and Herbal Compounds with Antiasthmatic Effect.
Rajizadeh MA, Najafipour H, Bejeshk MA. Rajizadeh MA, et al. Evid Based Complement Alternat Med. 2024 Feb 8;2024:5373117. doi: 10.1155/2024/5373117. eCollection 2024. Evid Based Complement Alternat Med. 2024. PMID: 39263346 Free PMC article. Review. - Effects of Ginger (Zingiber officinale) on the Hallmarks of Aging.
Matin M, Joshi T, Wang D, Tzvetkov NT, Matin FB, Wierzbicka A, Jóźwik A, Horbańczuk JO, Atanasov AG. Matin M, et al. Biomolecules. 2024 Aug 2;14(8):940. doi: 10.3390/biom14080940. Biomolecules. 2024. PMID: 39199328 Free PMC article. Review. - Network pharmacology, molecular docking, and dynamics analyses to predict the antiviral activity of ginger constituents against coronavirus infection.
Samy A, Hassan A, Hegazi NM, Farid M, Elshafei M. Samy A, et al. Sci Rep. 2024 May 27;14(1):12059. doi: 10.1038/s41598-024-60721-3. Sci Rep. 2024. PMID: 38802394 Free PMC article. - Performance of ginger constituents against SARS-CoV-2 virus: A therapeutic and theoretical approach.
Kadhim MM, Khadom AA, Abaies JK, Kadhum WR, Hachim SK. Kadhim MM, et al. Parasite Epidemiol Control. 2024 Apr 8;25:e00347. doi: 10.1016/j.parepi.2024.e00347. eCollection 2024 May. Parasite Epidemiol Control. 2024. PMID: 38629055 Free PMC article.
References
- Singh BB, Khorsan R, Vinjamury SP, Der-Martirosian C, Kizhakkeveettil A, Anderson TM. Herbal treatments of asthma: a systematic review. J Asthma 2007;44:685–698 - PubMed
- Kao ST, Chang CH, Chen YS, Chiang SY, Lin JG. Effects of Ding-Chuan-Tang on bronchoconstriction and airway leucocyte infiltration in sensitized guinea pigs. Immunopharmacol Immunotoxicol 2004;26:113–124 - PubMed
- Li A, Xie Y, Qi F, Li J, Wang P, Xu S, Zhao L. Anti-virus effect of traditional Chinese medicine Yi-Fu-Qing granule on acute respiratory tract infections. Biosci Trends. 2009;3:119–123 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous