Mitochondria and mitophagy: the yin and yang of cell death control - PubMed (original) (raw)
Review
Mitochondria and mitophagy: the yin and yang of cell death control
Dieter A Kubli et al. Circ Res. 2012.
Abstract
Mitochondria are primarily responsible for providing the contracting cardiac myocyte with a continuous supply of ATP. However, mitochondria can rapidly change into death-promoting organelles. In response to changes in the intracellular environment, mitochondria become producers of excessive reactive oxygen species and release prodeath proteins, resulting in disrupted ATP synthesis and activation of cell death pathways. Interestingly, cells have developed a defense mechanism against aberrant mitochondria that can cause harm to the cell. This mechanism involves selective sequestration and subsequent degradation of the dysfunctional mitochondrion before it causes activation of cell death. Induction of mitochondrial autophagy, or mitophagy, results in selective clearance of damaged mitochondria in cells. In response to stress such as ischemia/reperfusion, prosurvival and prodeath pathways are concomitantly activated in cardiac myocytes. Thus, there is a delicate balance between life and death in the myocytes during stress, and the final outcome depends on the complex cross-talk between these pathways. Mitophagy functions as an early cardioprotective response, favoring adaptation to stress by removing damaged mitochondria. In contrast, increased oxidative stress and apoptotic proteases can inactivate mitophagy, allowing for the execution of cell death. Herein, we discuss the importance of mitochondria and mitophagy in cardiovascular health and disease and provide a review of our current understanding of how these processes are regulated.
Figures
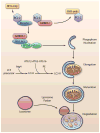
Figure 1
Induction of autophagy. BCL-2/BCL-XL prevents induction of autophagy by binding BECLIN1 and AMBRA1. Displacement by BH3-only proteins leads to activation of the BECLIN1-VPS34-VPS15 complex and phagophore nucleation. Elongation of the membrane requires two ubiquitin-like conjugation systems: ATG12-ATG5-ATG16L, and conjugation of LC3-I with phosphatidylethanolamine (PE) to form LC3-II. After maturation of the autophagosome, it fuses with the lysosome to degrade the cargo. (Illustration Credit: Ben Smith).
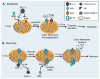
Figure 2
Mechanisms of mitochondrial membrane permeabilization. A. Anti-apoptotic BCL-2 family proteins, such as BCL-2 and BCL-XL, inhibit BAX/BAK-mediated outer mitochondrial membrane permeabilization. BAX/BAK activation causes release of cytotoxic proteins, slow loss of DYm, and culminates in apoptosis. B. Opening of the mPTP results in rapid influx of solutes and water which causes dissipation of the Dym, inner membrane swelling and subsequent outer membrane rupture. Destruction of the outer membrane releases cytotoxic proteins into the cytosol and leads to necrotic cell death. Anti-apoptotic BCL-2 proteins also prevent mPTP opening, while pro-apoptotic proteins such as Bax can enhance mPTP opening.
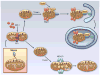
Figure 3
Regulation of mitophagy. Damaged mitochondria undergo DRP1-mediated fission prior to mitophagy. Reduced mitochondrial membrane potential leads to accumulation of PINK1 and subsequent recruitment of the E3 ubiquitin ligase Parkin to mitochondria. Parkin promotes ubiquitination of proteins in the mitochondrial membrane, which targets the damaged mitochondrion for removal by an autophagosome. The healthy mitochondrial fragment will undergo fusion mediated by MFN1/2 and OPA1. (Illustration Credit: Ben Smith).
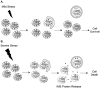
Figure 4
Removal of mitochondria via autophagy adaptors or autophagy receptors. A. The p62 protein interacts with ubiquitinated proteins on the mitochondrion. The complex is then selectively sequestered by an autophagosome through the interaction between p62 and LC3. B. Autophagy receptors on the mitochondria such as Bnip3 and Nix interact directly with LC3 on the autophagosome. (Illustration Credit: Ben Smith).
Figure 5
Balance between life and death during stress. A. Mild stress causes damage to a few mitochondria, which are rapidly sequestered by autophagosomes. B. In response to severe stress, there is overwhelming mitochondrial damage that autophagosomes are unable to efficiently clear. These mitochondria will release pro-death proteins from the intermembrane space (IMS), such as cytochrome c, AIF, and SMAC/Diablo, that will activate cell death pathways.
Similar articles
- Mitochondrial autophagy and cell survival is regulated by the circadian Clock gene in cardiac myocytes during ischemic stress.
Rabinovich-Nikitin I, Rasouli M, Reitz CJ, Posen I, Margulets V, Dhingra R, Khatua TN, Thliveris JA, Martino TA, Kirshenbaum LA. Rabinovich-Nikitin I, et al. Autophagy. 2021 Nov;17(11):3794-3812. doi: 10.1080/15548627.2021.1938913. Epub 2021 Aug 7. Autophagy. 2021. PMID: 34085589 Free PMC article. - Overexpression of Rcan1-1L inhibits hypoxia-induced cell apoptosis through induction of mitophagy.
Sun L, Hao Y, An R, Li H, Xi C, Shen G. Sun L, et al. Mol Cells. 2014 Nov;37(11):785-94. doi: 10.14348/molcells.2014.0103. Epub 2014 Nov 5. Mol Cells. 2014. PMID: 25377251 Free PMC article. - Oxidative stress-induced autophagy in plants: the role of mitochondria.
Minibayeva F, Dmitrieva S, Ponomareva A, Ryabovol V. Minibayeva F, et al. Plant Physiol Biochem. 2012 Oct;59:11-9. doi: 10.1016/j.plaphy.2012.02.013. Epub 2012 Feb 16. Plant Physiol Biochem. 2012. PMID: 22386760 Review. - Mitochondrial quality surveillance: mitophagy in cardiovascular health and disease.
Diao RY, Gustafsson ÅB. Diao RY, et al. Am J Physiol Cell Physiol. 2022 Feb 1;322(2):C218-C230. doi: 10.1152/ajpcell.00360.2021. Epub 2021 Dec 29. Am J Physiol Cell Physiol. 2022. PMID: 34965154 Free PMC article. Review. - Mitophagy regulates mitochondrial network signaling, oxidative stress, and apoptosis during myoblast differentiation.
Baechler BL, Bloemberg D, Quadrilatero J. Baechler BL, et al. Autophagy. 2019 Sep;15(9):1606-1619. doi: 10.1080/15548627.2019.1591672. Epub 2019 Apr 7. Autophagy. 2019. PMID: 30859901 Free PMC article.
Cited by
- Brief Report: Oxidative Stress Mediates Cardiomyocyte Apoptosis in a Human Model of Danon Disease and Heart Failure.
Hashem SI, Perry CN, Bauer M, Han S, Clegg SD, Ouyang K, Deacon DC, Spinharney M, Panopoulos AD, Izpisua Belmonte JC, Frazer KA, Chen J, Gong Q, Zhou Z, Chi NC, Adler ED. Hashem SI, et al. Stem Cells. 2015 Jul;33(7):2343-50. doi: 10.1002/stem.2015. Epub 2015 May 13. Stem Cells. 2015. PMID: 25826782 Free PMC article. - Salidroside Ameliorates Mitochondria-Dependent Neuronal Apoptosis after Spinal Cord Ischemia-Reperfusion Injury Partially through Inhibiting Oxidative Stress and Promoting Mitophagy.
Gu C, Li L, Huang Y, Qian D, Liu W, Zhang C, Luo Y, Zhou Z, Kong F, Zhao X, Liu H, Gao P, Chen J, Yin G. Gu C, et al. Oxid Med Cell Longev. 2020 Jul 23;2020:3549704. doi: 10.1155/2020/3549704. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32774670 Free PMC article. - The Effects of Methylene Blue on Autophagy and Apoptosis in MRI-Defined Normal Tissue, Ischemic Penumbra and Ischemic Core.
Jiang Z, Watts LT, Huang S, Shen Q, Rodriguez P, Chen C, Zhou C, Duong TQ. Jiang Z, et al. PLoS One. 2015 Jun 29;10(6):e0131929. doi: 10.1371/journal.pone.0131929. eCollection 2015. PLoS One. 2015. PMID: 26121129 Free PMC article. - Diatom-Derived Polyunsaturated Aldehydes Activate Similar Cell Death Genes in Two Different Systems: Sea Urchin Embryos and Human Cells.
Galasso C, Celentano S, Costantini M, D'Aniello S, Ianora A, Sansone C, Romano G. Galasso C, et al. Int J Mol Sci. 2020 Jul 22;21(15):5201. doi: 10.3390/ijms21155201. Int J Mol Sci. 2020. PMID: 32708040 Free PMC article. - The ER stress-mediated mitochondrial apoptotic pathway and MAPKs modulate tachypacing-induced apoptosis in HL-1 atrial myocytes.
Shi J, Jiang Q, Ding X, Xu W, Wang DW, Chen M. Shi J, et al. PLoS One. 2015 Feb 17;10(2):e0117567. doi: 10.1371/journal.pone.0117567. eCollection 2015. PLoS One. 2015. PMID: 25689866 Free PMC article.
References
- Schaper J, Meiser E, Stammler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res. 1985;56:377–391. - PubMed
- Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–2812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL087023/HL/NHLBI NIH HHS/United States
- R01HL101217/HL/NHLBI NIH HHS/United States
- R01HL092136/HL/NHLBI NIH HHS/United States
- R01 HL101217/HL/NHLBI NIH HHS/United States
- R01 HL092136/HL/NHLBI NIH HHS/United States
- R01HL087023/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources