Evolution of apicomplexan secretory organelles - PubMed (original) (raw)
Review
Evolution of apicomplexan secretory organelles
Marc-Jan Gubbels et al. Int J Parasitol. 2012 Nov.
Abstract
The alveolate superphylum includes many free-living and parasitic organisms, which are united by the presence of alveolar sacs lying proximal to the plasma membrane, providing cell structure. All species comprising the apicomplexan group of alveolates are parasites and have adapted to the unique requirements of the parasitic lifestyle. Here the evolution of apicomplexan secretory organelles that are involved in the critical process of egress from one cell and invasion of another is explored. The variations within the Apicomplexa and how these relate to species-specific biology will be discussed. In addition, recent studies have identified specific calcium-sensitive molecules that coordinate the various events and regulate the release of these secretory organelles within apicomplexan parasites. Some aspects of this machinery are conserved outside the Apicomplexa, and are beginning to elucidate the conserved nature of the machinery. Briefly, the relationship of this secretion machinery within the Apicomplexa will be discussed, compared with free-living and predatory alveolates, and how these might have evolved from a common ancestor.
Copyright © 2012 Australian Society for Parasitology Inc. Published by Elsevier Ltd. All rights reserved.
Figures
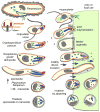
Fig 1
Schematic comparison of organelles with a role in apicomplexan host cell invasion across the Alveolates. The surface of the free-living ciliate, Paramecium, is covered by cilia which it uses for swimming. Prey bacteria (light green) are taken up through the oral cavity by phagocytosis (green arrow) and merge with lysomes in the cytoplasm for digestion. The enlargement shows the alternating trichocysts (or dense core secretory vesicles (DCSVs)) and cilia underlying the plasma membrane and protruding from the alveolar vesicles. Colpodella vorax is a representative of a dinoflagellate lineage with two flagella that feeds by myzocytosis, also known as cellular vampirism. Note the open conoid structure that is in close apposition upon attachment to a prey cell (flagellate protists). Rhoptries and micronemes are secreted in the process. Prey cell cytoplasm is taken up by pinocytosis and accumulates in basally located vacuoles. Cryptosporidium parvum is an apicomplexan parasite closely related to the archigregarine lineage. A gliding motile sporozoite (1) is shown attaching with its apical end to an endothelial cell of a vertebrate host. Host actin polymerization is induced by the parasite and triggers pseudopod formation, which will engulf the parasite (3), as well as inducing an actin patch, keeping the parasite at the edge of the host cell. This results in extracytoplasmic, yet intracellular, residence of the vacuole. Note the single membrane separating the parasite and host cell cytoplasm, known as the feeder organelle, which is reminiscent of myzocytosis. Toxoplasma gondii tachyzoites and P. falciparum sporozoites display gliding motility, which drives invasion of vertebrate cells. A constriction known as the moving junction forms at the interface of the parasite and the host, and excludes plasma membrane proteins from the host entering into the parasitophorous vacuole membrane. Plasmodium falciparum merozoites enter red blood cells by a combination of gliding motility and zippering. Theileria sporozoites or merozoites are non-motile and can enter host cells in any orientation, a process whereby the dense coat of the zoite is shed. Evidence for micronemes has not been clearly established while no moving junction is formed and the rhoptries are only released upon completion of invasion. Secreted rhoptry and dense granule proteins dissolve the vacuolar membrane and results in cytoplasmic residence. Orange: alveoli or inner membrane complex (IMC); red: micronemes (trichocysts or DSCVs in Paramecium); blue: rhoptries (lysosomes in Paramecium); dark green: dense granules; yellow: conoid. Parasite cells are not drawn to scale.
Similar articles
- Molecular and functional aspects of parasite invasion.
Soldati D, Foth BJ, Cowman AF. Soldati D, et al. Trends Parasitol. 2004 Dec;20(12):567-74. doi: 10.1016/j.pt.2004.09.009. Trends Parasitol. 2004. PMID: 15522666 Review. - Cryptic organelle homology in apicomplexan parasites: insights from evolutionary cell biology.
Klinger CM, Nisbet RE, Ouologuem DT, Roos DS, Dacks JB. Klinger CM, et al. Curr Opin Microbiol. 2013 Aug;16(4):424-31. doi: 10.1016/j.mib.2013.07.015. Epub 2013 Aug 8. Curr Opin Microbiol. 2013. PMID: 23932202 Free PMC article. - A genome-wide analysis of coatomer protein (COP) subunits of apicomplexan parasites and their evolutionary relationships.
Kibria KMK, Ferdous J, Sardar R, Panda A, Gupta D, Mohmmed A, Malhotra P. Kibria KMK, et al. BMC Genomics. 2019 Jan 31;20(1):98. doi: 10.1186/s12864-019-5463-1. BMC Genomics. 2019. PMID: 30704415 Free PMC article. - The apicoplast: a red alga in human parasites.
Striepen B. Striepen B. Essays Biochem. 2011;51:111-25. doi: 10.1042/bse0510111. Essays Biochem. 2011. PMID: 22023445 Review. - Apicomplexa Cell Cycles: Something Old, Borrowed, Lost, and New.
White MW, Suvorova ES. White MW, et al. Trends Parasitol. 2018 Sep;34(9):759-771. doi: 10.1016/j.pt.2018.07.006. Epub 2018 Aug 2. Trends Parasitol. 2018. PMID: 30078701 Free PMC article. Review.
Cited by
- Protozoan phagotrophy from predators to parasites: An overview of the enigmatic cytostome-cytopharynx complex of Trypanosoma cruzi.
Etheridge RD. Etheridge RD. J Eukaryot Microbiol. 2022 Nov;69(6):e12896. doi: 10.1111/jeu.12896. Epub 2022 Mar 8. J Eukaryot Microbiol. 2022. PMID: 35175673 Free PMC article. Review. - Epiplasts: Membrane Skeletons and Epiplastin Proteins in Euglenids, Glaucophytes, Cryptophytes, Ciliates, Dinoflagellates, and Apicomplexans.
Goodenough U, Roth R, Kariyawasam T, He A, Lee JH. Goodenough U, et al. mBio. 2018 Oct 30;9(5):e02020-18. doi: 10.1128/mBio.02020-18. mBio. 2018. PMID: 30377285 Free PMC article. - Identification of host protein ENO1 (alpha-enolase) interacting with Cryptosporidium parvum sporozoite surface protein, Cpgp40.
Wang Y, Li N, Liang G, Wang L, Zhang X, Cui Z, Li X, Zhang S, Zhang L. Wang Y, et al. Parasit Vectors. 2024 Mar 19;17(1):146. doi: 10.1186/s13071-024-06233-5. Parasit Vectors. 2024. PMID: 38504274 Free PMC article. - Lytic Cycle of Toxoplasma gondii: 15 Years Later.
Blader IJ, Coleman BI, Chen CT, Gubbels MJ. Blader IJ, et al. Annu Rev Microbiol. 2015;69:463-85. doi: 10.1146/annurev-micro-091014-104100. Epub 2015 Aug 28. Annu Rev Microbiol. 2015. PMID: 26332089 Free PMC article. Review. - Two Phosphoglucomutase Paralogs Facilitate Ionophore-Triggered Secretion of the Toxoplasma Micronemes.
Saha S, Coleman BI, Dubey R, Blader IJ, Gubbels MJ. Saha S, et al. mSphere. 2017 Nov 29;2(6):e00521-17. doi: 10.1128/mSphere.00521-17. eCollection 2017 Nov-Dec. mSphere. 2017. PMID: 29202046 Free PMC article.
References
- Allen RD, Fok AK. Membrane trafficking and processing in Paramecium. Int Rev Cytol. 2000:198. - PubMed
- Barta JR, Thompson RC. What is Cryptosporidium? Reappraising its biology and phylogenetic affinities. Trends Parasitol. 2006;22:463–468. - PubMed
- Billker O, Dechamps S, Tewari R, Wenig G, Franke-Fayard B, Brinkmann V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell. 2004;117:503–514. - PubMed
Publication types
MeSH terms
Grants and funding
- AI057919/AI/NIAID NIH HHS/United States
- AI088314/AI/NIAID NIH HHS/United States
- R01 AI057919/AI/NIAID NIH HHS/United States
- R21 AI088314/AI/NIAID NIH HHS/United States
- RRP 12-175/HX/HSRD VA/United States
- R21 AI081220/AI/NIAID NIH HHS/United States
- AI081220/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources