c-Myc and cancer metabolism - PubMed (original) (raw)
c-Myc and cancer metabolism
Donald M Miller et al. Clin Cancer Res. 2012.
Abstract
The processes of cellular growth regulation and cellular metabolism are closely interrelated. The c-Myc oncogene is a "master regulator" which controls many aspects of both of these processes. The metabolic changes which occur in transformed cells, many of which are driven by c-Myc overexpression, are necessary to support the increased need for nucleic acids, proteins, and lipids necessary for rapid cellular proliferation. At the same time, c-Myc overexpression results in coordinated changes in level of expression of gene families which result in increased cellular proliferation. This interesting duality of c-Myc effects places it in the mainstream of transformational changes and gives it a very important role in regulating the "transformed phenotype." The effects induced by c-Myc can occur either as a "primary oncogene" which is activated by amplification or translocation or as a downstream effect of other activated oncogenes. In either case, it appears that c-Myc plays a central role in sustaining the changes which occur with transformation. Although efforts to use c-Myc as a therapeutic target have been quite frustrating, it appears that this may change in the next few years.
©2012 AACR
Conflict of interest statement
Dr. Miller is on the Board of Directors of Advanced Cancer Therapeutics; All other authors declare no potential conflict of interest.
Figures
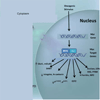
Figure 1
Diagram of Myc effects in transformed cells. There are a wide variety of downstream pathways which are both positively and negatively regulated by Myc expression.
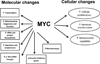
Figure 2
Pleiotropic Effects of c-Myc Expression. The c-myc gene has a variety of molecular and cellular effects (which are closely related). These effects result from myc-mediated changes in large gene families which drive cellular functions. Microarray studies have shown that these changes occur in concert and have major effects on cellular function.
Similar articles
- MYC-induced cancer cell energy metabolism and therapeutic opportunities.
Dang CV, Le A, Gao P. Dang CV, et al. Clin Cancer Res. 2009 Nov 1;15(21):6479-83. doi: 10.1158/1078-0432.CCR-09-0889. Epub 2009 Oct 27. Clin Cancer Res. 2009. PMID: 19861459 Free PMC article. Review. - Tumor suppressor NDRG2 inhibits glycolysis and glutaminolysis in colorectal cancer cells by repressing c-Myc expression.
Xu X, Li J, Sun X, Guo Y, Chu D, Wei L, Li X, Yang G, Liu X, Yao L, Zhang J, Shen L. Xu X, et al. Oncotarget. 2015 Sep 22;6(28):26161-76. doi: 10.18632/oncotarget.4544. Oncotarget. 2015. PMID: 26317652 Free PMC article. - Impact of MYC in regulation of tumor cell metabolism.
Wahlström T, Henriksson MA. Wahlström T, et al. Biochim Biophys Acta. 2015 May;1849(5):563-9. doi: 10.1016/j.bbagrm.2014.07.004. Epub 2014 Jul 17. Biochim Biophys Acta. 2015. PMID: 25038584 Review. - MYC, metabolism, cell growth, and tumorigenesis.
Dang CV. Dang CV. Cold Spring Harb Perspect Med. 2013 Aug 1;3(8):a014217. doi: 10.1101/cshperspect.a014217. Cold Spring Harb Perspect Med. 2013. PMID: 23906881 Free PMC article. - Rethinking the Warburg effect with Myc micromanaging glutamine metabolism.
Dang CV. Dang CV. Cancer Res. 2010 Feb 1;70(3):859-62. doi: 10.1158/0008-5472.CAN-09-3556. Epub 2010 Jan 19. Cancer Res. 2010. PMID: 20086171 Free PMC article. Review.
Cited by
- Identifying strategies to target the metabolic flexibility of tumours.
Méndez-Lucas A, Lin W, Driscoll PC, Legrave N, Novellasdemunt L, Xie C, Charles M, Wilson Z, Jones NP, Rayport S, Rodríguez-Justo M, Li V, MacRae JI, Hay N, Chen X, Yuneva M. Méndez-Lucas A, et al. Nat Metab. 2020 Apr;2(4):335-350. doi: 10.1038/s42255-020-0195-8. Epub 2020 Apr 21. Nat Metab. 2020. PMID: 32694609 Free PMC article. - Pancancer survival analysis of cancer hallmark genes.
Nagy Á, Munkácsy G, Győrffy B. Nagy Á, et al. Sci Rep. 2021 Mar 15;11(1):6047. doi: 10.1038/s41598-021-84787-5. Sci Rep. 2021. PMID: 33723286 Free PMC article. - Hepatic oxidative stress, up-regulation of pro-inflammatory cytokines, apoptotic and oncogenic markers following 2-methoxyethanol administrations in rats.
Somade OT, Ajayi BO, Olunaike OE, Jimoh LA. Somade OT, et al. Biochem Biophys Rep. 2020 Aug 29;24:100806. doi: 10.1016/j.bbrep.2020.100806. eCollection 2020 Dec. Biochem Biophys Rep. 2020. PMID: 32913901 Free PMC article. - Ethylene glycol monomethyl ether-induced testicular oxidative stress and time-dependent up-regulation of apoptotic, pro-inflammatory, and oncogenic markers in rats.
Somade OT, Ajayi BO, Adeyi OE, Adeshina AA, James AS, Ayodele PF. Somade OT, et al. Metabol Open. 2020 Aug 17;7:100051. doi: 10.1016/j.metop.2020.100051. eCollection 2020 Sep. Metabol Open. 2020. PMID: 32924002 Free PMC article. - PKM2, a Central Point of Regulation in Cancer Metabolism.
Wong N, De Melo J, Tang D. Wong N, et al. Int J Cell Biol. 2013;2013:242513. doi: 10.1155/2013/242513. Epub 2013 Feb 14. Int J Cell Biol. 2013. PMID: 23476652 Free PMC article.
References
- Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. - PubMed
- Lombardi L, Newcomb EW, Dalla-Favera R. Pathogenesis of Burkitt lymphoma: expression of an activated c-myc oncogene causes the tumorigenic conversion of EBV-infected human B lymphoblasts. Cell. 1987;49:161–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR018733/RR/NCRR NIH HHS/United States
- P20 GM103482/GM/NIGMS NIH HHS/United States
- P30 GM106396/GM/NIGMS NIH HHS/United States
- 3P20RR18733/RR/NCRR NIH HHS/United States
- R21 CA191663/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources