The role of glycogen synthase kinase-3 signaling in neurodevelopment and fragile X syndrome - PubMed (original) (raw)
The role of glycogen synthase kinase-3 signaling in neurodevelopment and fragile X syndrome
Samantha Portis et al. Int J Physiol Pathophysiol Pharmacol. 2012.
Abstract
Fragile X syndrome (FXS), one of the most common genetic causes of autism, results from a loss of fragile X mental retardation protein (FMRP) expression. At the molecular level, abnormal neurodevelopment is thought to result from dysregulated protein synthesis of key neural synaptic proteins, however recent evidence suggests broader roles for this protein including glutamate signaling, memory, and regulation of the critical serine/threonine regulatory kinase, glycogen synthase kinase-3 (GSK-3). In this review, genetic and molecular features of FXS are detailed in the context of FXS neuropathology. Additionally, potential mechanisms by which FMRP silencing impacts GSK-3 and GSK-3-associated signaling pathways are discussed. As GSK-3 signaling represents a central regulatory node for critical neurodevelopmental pathways, understanding how FXS results from FMRP-mediated GSK-3 dysregulation may provide novel therapeutic targets for disease-modifying interventions for FXS and related ASDs.
Keywords: Glycogen synthase kinase; flavonoids; fragile X; lithium; microglia; neuroinflammation; trinucucleotide repeat.
Figures
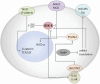
Figure 1
GSK3 regulates a variety of pathways involved in neurodevelopment. Active GSK3 regulates the canonical Wnt pathway by remaining bound to the Wnt/β-catenin destruction complex that includes APC and Axin. This complex targets β-catenin for proteasomal degradation. Inhibitory phosyphorylation of GSK3 releases β-catenin from the complex. It is then recruited to the nucleus by TCF/LEF where it induces gene transcription. GSK3β is known to phosphorylate and inhibit Notch, resulting in the inhibition of many Notch target genes. This inhibitory phosphorylation is reversed when Wnt1 is present. Inhibitory phosphorylation is induced by a number of kinases. The PI3/Akt pathway regulates GSK-3 activity by inducing inhibitory serine-phosphorylation. FMRP is deficient in Fragile X syndrome and is normally responsible for regulating mRNA transcription of metabotropic glutamate receptor-5 (mGluR5) and GSK3. It has been shown that inhibition of mGluR5 leads to the inhibition of GSK3. In addition to regulating neurodevelopmental pathways, it has been shown that GSK3 is also involved in pathways that promote inflammation. GSK3 induces IL-6 production, leading to the subsequent phosphorylation of Jak2/STAT3.
Similar articles
- Glycogen synthase kinase-3: a promising therapeutic target for fragile x syndrome.
Mines MA, Jope RS. Mines MA, et al. Front Mol Neurosci. 2011 Nov 1;4:35. doi: 10.3389/fnmol.2011.00035. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22053151 Free PMC article. - microRNAs and Fragile X Syndrome.
Lin SL. Lin SL. Adv Exp Med Biol. 2015;888:107-21. doi: 10.1007/978-3-319-22671-2_7. Adv Exp Med Biol. 2015. PMID: 26663181 - Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome.
Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Gross C, et al. J Neurosci. 2010 Aug 11;30(32):10624-38. doi: 10.1523/JNEUROSCI.0402-10.2010. J Neurosci. 2010. PMID: 20702695 Free PMC article. - Aberrant RNA translation in fragile X syndrome: From FMRP mechanisms to emerging therapeutic strategies.
Banerjee A, Ifrim MF, Valdez AN, Raj N, Bassell GJ. Banerjee A, et al. Brain Res. 2018 Aug 15;1693(Pt A):24-36. doi: 10.1016/j.brainres.2018.04.008. Epub 2018 Apr 10. Brain Res. 2018. PMID: 29653083 Free PMC article. Review. - Melatonin as a Novel Interventional Candidate for Fragile X Syndrome with Autism Spectrum Disorder in Humans.
Won J, Jin Y, Choi J, Park S, Lee TH, Lee SR, Chang KT, Hong Y. Won J, et al. Int J Mol Sci. 2017 Jun 20;18(6):1314. doi: 10.3390/ijms18061314. Int J Mol Sci. 2017. PMID: 28632163 Free PMC article. Review.
Cited by
- Role of phosphodiesterases in the pathophysiology of neurodevelopmental disorders.
Delhaye S, Bardoni B. Delhaye S, et al. Mol Psychiatry. 2021 Sep;26(9):4570-4582. doi: 10.1038/s41380-020-00997-9. Epub 2021 Jan 7. Mol Psychiatry. 2021. PMID: 33414502 Free PMC article. Review. - Critical Effects on Akt Signaling in Adult Zebrafish Brain Following Alterations in Light Exposure.
Moore NS, Mans RA, McCauley MK, Allgood CS, Barksdale KA. Moore NS, et al. Cells. 2021 Mar 12;10(3):637. doi: 10.3390/cells10030637. Cells. 2021. PMID: 33809219 Free PMC article. - Molecular Mechanisms of Synaptic Dysregulation in Fragile X Syndrome and Autism Spectrum Disorders.
Telias M. Telias M. Front Mol Neurosci. 2019 Mar 7;12:51. doi: 10.3389/fnmol.2019.00051. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30899214 Free PMC article. Review. - Putative Mechanisms of Action and Clinical Use of Lithium in Children and Adolescents: A Critical Review.
Pisano S, Pozzi M, Catone G, Scrinzi G, Clementi E, Coppola G, Milone A, Bravaccio C, Santosh P, Masi G. Pisano S, et al. Curr Neuropharmacol. 2019;17(4):318-341. doi: 10.2174/1570159X16666171219142120. Curr Neuropharmacol. 2019. PMID: 29256353 Free PMC article. Review. - Pharmacotherapy for Fragile X Syndrome: Progress to Date.
Davenport MH, Schaefer TL, Friedmann KJ, Fitzpatrick SE, Erickson CA. Davenport MH, et al. Drugs. 2016 Mar;76(4):431-45. doi: 10.1007/s40265-016-0542-y. Drugs. 2016. PMID: 26858239 Review.
References
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. - PubMed
- Koukoui SD, Chaudhuri A. Neuroanatomical, molecular genetic, and behavioral correlates of fragile X syndrome. Brain Res Rev. 2007;53:27–38. - PubMed
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9:1221–1225. - PubMed
- Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: a model for autism and neurodegeneration. Curr Opin Psychiatry. 2005;18:490–496. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials