Transcriptional control of effector and memory CD8+ T cell differentiation - PubMed (original) (raw)
Review
. 2012 Nov;12(11):749-61.
doi: 10.1038/nri3307. Epub 2012 Oct 19.
Affiliations
- PMID: 23080391
- PMCID: PMC4137483
- DOI: 10.1038/nri3307
Review
Transcriptional control of effector and memory CD8+ T cell differentiation
Susan M Kaech et al. Nat Rev Immunol. 2012 Nov.
Abstract
During an infection, T cells can differentiate into multiple types of effector and memory T cells, which help to mediate pathogen clearance and provide long-term protective immunity. These cells can vary in their phenotype, function and location, and in their long-term fate in terms of their ability to populate the memory T cell pool. Over the past decade, the signalling pathways and transcriptional programmes that regulate the formation of heterogeneous populations of effector and memory CD8(+) T cells have started to be characterized, and this Review discusses the major advances in these areas.
Figures
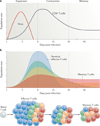
Figure 1. Kinetics of a T cell response and distribution of memory cell potential
a | During an acute viral infection, antigen-specific T cells rapidly proliferate (during the expansion phase) and differentiate into cytotoxic T lymphocytes (CTLs) that mediate viral clearance. Most of these cells die over the next several weeks during the contraction phase of the response. Only a small percentage of effector T cells (5–10%) survive and further develop into functional mature memory CD8+ T cells. b | The pool of effector T cells can be separated into multiple diverse subsets based on differences in gene and protein expression, effector functions, migratory patterns, proliferative capacity and long-term fate. Ultimately, not all effector T cells have equal potential to form memory T cells. Some cell-surface markers correlate with distinct effector and memory T cell fates: terminal effector T cells (shown in blue) are KLRG1hiIL-7RαlowCD27lowBCL-2low, and long-lived memory (and memory precursor) cells (shown in red) are KLRG1lowIL-7RαhiCD27hiBCL-2hi. However, other T cell subsets with intermediate differentiation states also exist that have mixed phenotypes, longevities and abilities to self-renew, as depicted by the yellow and green populations. Over time, there may also be some interconversion between these subsets.
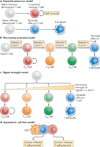
Figure 2. Models for generating effector and memory T cell heterogeneity
a | In the separate-precursor model, naive T cells are ‘pre-programmed’ to adopt certain differentiation states following activation based on information received during thymic development. However, currently, little evidence exists to support this model. b | The decreasing-potential model suggests that effector T cells adopt various differentiation states according to the cumulative history of signals that they encounter during infection. Repetitive stimulation with antigen and other signals, such as interleukin-2 (IL-2) and IL-12, drives greater effector cell proliferation and terminal differentiation. As the cells acquire terminally differentiated states they remain functional and cytolytic but lose memory cell properties, such as enhanced longevity and proliferative potential. c | In the signal-strength model, the formation of heterogeneous effector cell populations is dependent on the overall ‘strength’ of the signals (signals 1, 2 and 3 denote antigen, co-stimulatory molecules and cytokines, respectively) that are encountered early during T cell priming. In combination, a strong signal may drive greater clonal expansion and be important for selecting out T cells that are competent to form memory cells, but when delivered in excess these signals may also cause terminal effector T cell differentiation. d | The asymmetric cell fate model suggests that effector and memory T cell fates can arise from a single precursor T cell through asymmetric cell division that occurs as early as the first cell division after antigen stimulation. Evidence suggests that the proximal daughter cell (which is closer to the antigen-presenting cell (APC)) adopts an effector cell fate, whereas the distal daughter cell (which is further from the APC) adopts a memory cell fate. TCM, central memory T; TEM, effector memory T; TTM, transitional memory T.
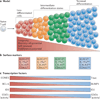
Figure 3. Graded activity of transcriptional programmes that control effector and memory T cell differentiation
a | This model postulates that, in response to different levels of signal input, the differentiation of antigen-specific effector CD8+ T cells occurs along a continuum. Cells that have greater memory cell potential, longevity and proliferative potential are at one end of the spectrum, and terminally differentiated effector T cells are present at the other end of the spectrum. In between these two extreme end points are effector T cells that exist in intermediate differentiation states. b | The heterogeneous differentiation states of effector CD8+ T cells can be distinguished by the expression of several surface markers, such as killer cell lectin-like receptor G1 (KLRG1), IL-7 receptor subunit-α (IL-7Rα), CD27, CXC-chemokine receptor 3 (CXCR3) and CD62L. c | The transcriptional programmes that control terminal effector cell differentiation and memory cell potential seem to be based on the graded expression or activity of certain competing sets of transcription factors. As the relative expression or activity of T-bet, B lymphocyte-induced maturation protein 1 (BLIMP1), inhibitor of DNA binding 2 (ID2) and signal transducer and activator of transcription 4 (STAT4) increases and surpasses a given threshold during infection, effector CD8+ T cells acquire more terminally differentiated phenotypes that are associated with a reduction in proliferative capacity and longevity. These factors counter-regulate, and can also interact with, an opposing set of transcriptional regulators, including eomesodermin (EOMES), B cell lymphoma 6 (BCL-6), ID3 and STAT3, that prevent terminal differentiation of effector T cells and help to maintain memory cell properties. Note that to some extent this figure is a conceptual diagram, as the exact amounts of the different transcription factors or their activities have not been accurately measured in all cases.
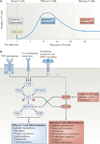
Figure 4. Model for the metabolic regulation of effector and memory T cell differentiation
a | Resting naive or memory T cells primarily generate ATP through fatty acid oxidation and mitochondrial respiration (which are catabolic processes), but following activation the T cells undergo a metabolic switch to lipid synthesis (an anabolic process) and aerobic glycolysis to meet the bioenergetic and biosynthetic demands for rapid clonal expansion and the production of effector molecules. Following pathogen clearance, it has been proposed that effector CD8+ T cells reduce their dependence on glycolysis and are gradually ‘reset’ back to a more catabolic state to survive and further develop into memory CD8+ T cells. b | In response to T cell receptor (TCR), co-stimulatory and cytokine signals, the activity of the phosphoinositide 3-kinase (PI3K)–AKT–mammalian target of rapamycin (mTOR) pathway has a key role in regulating effector CD8+ T cell metabolism and differentiation by orchestrating nutrient uptake, protein translation and lipid synthesis in rapidly proliferating effector T cells. Typically AKT functions upstream of mTOR complex 1 (mTORC1) and downstream of mTORC2, but a recent study has shown that S6 kinase (S6K) can be activated independently of AKT in CD8+ T cells. By contrast, cellular stress and ATP deprivation (that is, an increased AMP to ATP ratio) activate AMP-activated protein kinase (AMPK), which in turn inhibits mTOR and enhances fatty acid oxidation to cope with limiting resources. In addition, these pathways can modulate effector T cell fate decisions through transcriptional regulation. Sustained PI3K–AKT– mTOR activity inhibits forkhead box O1 (FOXO1), which acts as a molecular switch to simultaneously induce T-bet and repress eomesodermin (EOMES) expression, and thereby promotes the clonal expansion and terminal differentiation of effector T cells. 4EBP, eIF4E–binding protein; PDK1, 3-phosphoinositide-dependent protein kinase 1.
Similar articles
- Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation.
Rutishauser RL, Kaech SM. Rutishauser RL, et al. Immunol Rev. 2010 May;235(1):219-33. doi: 10.1111/j.0105-2896.2010.00901.x. Immunol Rev. 2010. PMID: 20536566 Review. - Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation.
Best JA, Blair DA, Knell J, Yang E, Mayya V, Doedens A, Dustin ML, Goldrath AW; Immunological Genome Project Consortium. Best JA, et al. Nat Immunol. 2013 Apr;14(4):404-12. doi: 10.1038/ni.2536. Epub 2013 Feb 10. Nat Immunol. 2013. PMID: 23396170 Free PMC article. - The interface between transcriptional and epigenetic control of effector and memory CD8⁺ T-cell differentiation.
Gray SM, Kaech SM, Staron MM. Gray SM, et al. Immunol Rev. 2014 Sep;261(1):157-68. doi: 10.1111/imr.12205. Immunol Rev. 2014. PMID: 25123283 Free PMC article. Review. - Surviving the crash: transitioning from effector to memory CD8+ T cell.
D'Cruz LM, Rubinstein MP, Goldrath AW. D'Cruz LM, et al. Semin Immunol. 2009 Apr;21(2):92-8. doi: 10.1016/j.smim.2009.02.002. Epub 2009 Mar 6. Semin Immunol. 2009. PMID: 19269192 Free PMC article. Review. - Cheating the Hunger Games; Mechanisms Controlling Clonal Diversity of CD8 Effector and Memory Populations.
Kavazović I, Polić B, Wensveen FM. Kavazović I, et al. Front Immunol. 2018 Nov 29;9:2831. doi: 10.3389/fimmu.2018.02831. eCollection 2018. Front Immunol. 2018. PMID: 30555492 Free PMC article. Review.
Cited by
- Epigenetics behind CD8+ T cell activation and exhaustion.
Zu H, Chen X. Zu H, et al. Genes Immun. 2024 Nov 14. doi: 10.1038/s41435-024-00307-1. Online ahead of print. Genes Immun. 2024. PMID: 39543311 Review. - The role of mitochondrial transfer in the suppression of CD8+ T cell responses by Mesenchymal stem cells.
Vaillant L, Akhter W, Nakhle J, Simon M, Villalba M, Jorgensen C, Vignais ML, Hernandez J. Vaillant L, et al. Stem Cell Res Ther. 2024 Nov 4;15(1):394. doi: 10.1186/s13287-024-03980-1. Stem Cell Res Ther. 2024. PMID: 39497203 Free PMC article. - Methylmalonic acid induces metabolic abnormalities and exhaustion in CD8+ T cells to suppress anti-tumor immunity.
Tejero JD, Hesterberg RS, Drapela S, Ilter D, Raizada D, Lazure F, Kashfi H, Liu M, Silvane L, Avram D, Fernández-García J, Asara JM, Fendt SM, Cleveland JL, Gomes AP. Tejero JD, et al. Oncogene. 2024 Oct 29. doi: 10.1038/s41388-024-03191-1. Online ahead of print. Oncogene. 2024. PMID: 39472497 - Modified Dendritic cell-based T-cell expansion protocol and single-cell multi-omics allow for the selection of the most expanded and _in vitro_-effective clonotype via profiling of thousands of MAGE-A3-specific T-cells.
Sennikov S, Volynets M, Alrhmoun S, Perik-Zavodskii R, Perik-Zavodskaia O, Fisher M, Lopatnikova J, Shevchenko J, Nazarov K, Philippova J, Alsalloum A, Kurilin V, Silkov A. Sennikov S, et al. Front Immunol. 2024 Oct 10;15:1470130. doi: 10.3389/fimmu.2024.1470130. eCollection 2024. Front Immunol. 2024. PMID: 39450161 Free PMC article. - PD-1+ and TIM-3+ T cells widely express common γ-chain cytokine receptors in multiple myeloma patients, and IL-2, IL-7, IL-15 stimulation up-regulates PD-1 and TIM-3 on T cells.
Batorov EV, Ineshina AD, Aristova TA, Denisova VV, Sizikova SA, Batorova DS, Ushakova GY, Shevela EY, Chernykh ER. Batorov EV, et al. Oncol Res. 2024 Sep 18;32(10):1575-1587. doi: 10.32604/or.2024.047893. eCollection 2024. Oncol Res. 2024. PMID: 39308517 Free PMC article.
References
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
- Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nature Immunol. 2003;4:1191–1198. - PubMed
Publication types
MeSH terms
Grants and funding
- R01AI074699/AI/NIAID NIH HHS/United States
- R01 AI074699/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R21 AI081150/AI/NIAID NIH HHS/United States
- R21 AI097767/AI/NIAID NIH HHS/United States
- R21AI097767/AI/NIAID NIH HHS/United States
- R21AI081150/AI/NIAID NIH HHS/United States
- R01 AI066232/AI/NIAID NIH HHS/United States
- R01AI066232/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous