Visual contrast sensitivity in Alzheimer's disease, mild cognitive impairment, and older adults with cognitive complaints - PubMed (original) (raw)
Multicenter Study
. 2013 Apr;34(4):1133-44.
doi: 10.1016/j.neurobiolaging.2012.08.007. Epub 2012 Oct 18.
Darrell Wudunn, Susan M Pepin, Tamiko R MaGee, Brenna C McDonald, Laura A Flashman, Heather A Wishart, Heather S Pixley, Laura A Rabin, Nadia Paré, Jessica J Englert, Eben Schwartz, Joshua R Curtain, John D West, Darren P O'Neill, Robert B Santulli, Richard W Newman, Andrew J Saykin
Affiliations
- PMID: 23084085
- PMCID: PMC3545045
- DOI: 10.1016/j.neurobiolaging.2012.08.007
Multicenter Study
Visual contrast sensitivity in Alzheimer's disease, mild cognitive impairment, and older adults with cognitive complaints
Shannon L Risacher et al. Neurobiol Aging. 2013 Apr.
Abstract
Deficits in contrast sensitivity (CS) have been reported in Alzheimer's disease (AD). However, the extent of these deficits in prodromal AD stages, including mild cognitive impairment (MCI) or even earlier, has not been investigated. In this study, CS was assessed using frequency doubling technology in older adults with AD (n = 10), amnestic MCI (n = 28), cognitive complaints without performance deficits (CC; n = 20), and healthy controls (HC; n = 29). The association between CS and cognition was also evaluated. Finally, the accuracy of CS measures for classifying MCI versus HC was evaluated. CS deficits were found in AD and MCI, while CC showed intermediate performance between MCI and HC. Upper right visual field CS showed the most significant difference among groups. CS was also associated with cognitive performance. Finally, CS measures accurately classified MCI versus HC. The CS deficits in AD and MCI, and intermediate performance in CC, indicate that these measures are sensitive to early AD-associated changes. Therefore, frequency doubling technology-based measures of CS may have promise as a novel AD biomarker.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Figure 1
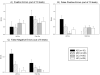
FDT-2 24-2 Performance Errors Measures of FDT-2 24-2 performance errors, including fixation errors (A), false positive errors (B) and false negative errors (C) are shown in Figure 1. False positive (B) and false negative (C) errors differed among groups in the left eye only (both p<0.05), with post-hoc comparisons indicating more false positive and false negative errors in AD patients relative to CC in the left eye (both p<0.05). See experimental procedures section for a description of how FDT-2 performance errors are tested. In all graphs, bars represent raw (unadjusted) mean performance errors +/− standard error. However, all statistical models (A-C) included age at visit, gender, and years of education as covariates.
Figure 2
FDT-2 Exam Duration and Mean Deviation and Pattern Standard Deviation in Visual Contrast Sensitivity Summary measures of visual contrast sensitivity (CS) performance, including exam duration (A), mean deviation (B), which is a measure of general CS, and pattern standard deviation (C), which measures the variability of CS across the retina, were significantly different among groups in both eyes (all p<0.001). See text for complete discussion of significant post-hoc comparisons. Briefly, AD patients showed impairment in nearly all FDT-2 test performance variables relative to CC and HC participants. In addition, MCI patients showed impairment in selected FDT-2 measures relative to HC participants. Bars represent adjusted group mean values +/− standard error (exam duration (A) was adjusted for age at visit, gender, and years of education; mean deviation (B) and pattern standard deviation (C) were adjusted for gender and years of education).
Figure 3
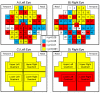
Group Differences in FDT-2 Contrast Sensitivity Thresholds across the Visual Field Significant differences among diagnostic groups were observed in contrast sensitivity (CS) thresholds in 93 of 110 regions across the visual field (VF) in the left (A) and right (B) eyes. In addition to evaluating single VF regions, mean contrast thresholds for four VF quadrants within each eye were assessed. All VF quadrants of the left (C) and right (D) eyes were significantly different among diagnostic groups. See text for discussion of between-group differences. All statistical models represented in this figure included age at visit, gender, and education as covariates.
Figure 4
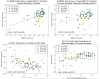
Relationship between Contrast Sensitivity and Cognition Significant associations between visual contrast sensitivity (CS) performance measured with the FDT-2 24-2 exam and cognitive performance were observed. General cognition (MMSE total score) was significantly associated with the mean bilateral contrast threshold of the upper right visual field (VF) quadrant (A). Upon visual inspection, one AD participant appeared to be a significant outlier and significantly more impaired, with an MMSE total score well below all other participants. However, when this participant was excluded (B), MMSE total score was still significantly associated with CS performance as measured by the mean bilateral contrast threshold of the upper right VF quadrant. Memory performance was also significantly associated with visual CS, including significant associations between CVLT total score and bilateral mean exam duration (C) and between CVLT long delay recall score and the mean deviation in CS (D). All participants were included in panels A, C, and D, while 1 AD participant was excluded (due to low MMSE total score) from panel B. Exclusion of the AD outlier did not affect the results in panels C and D. Psychometric and CS performance variables were adjusted for age at visit (all variables except for mean deviation), gender (all variables), and years of education (all variables). Different colors for each diagnostic group are included as indicated in the figure legend.
Figure 5
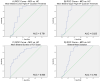
ROC Curves of MCI vs. HC Classification for Selected FDT-2 Variables Visual contrast sensitivity (CS) measures effectively classified patients with MCI and HC by diagnostic group. The bilateral mean contrast threshold in the lower right visual field (VF) quadrant (A) was the best classifier of MCI vs. HC, with an overall accuracy of 80.7% (specificity = 87.0%, sensitivity = 76.5%; AUC = 0.791). In addition, the bilateral mean contrast threshold in the upper right VF quadrant (B) successfully classified MCI vs. HC with an overall accuracy of 78.9% (specificity = 80.7%, sensitivity = 77.4%; AUC = 0.825). Summary measures of CS performance were also good classifiers, with bilateral mean exam duration (C) showing an overall accuracy of 71.5% (specificity = 70.0%, sensitivity = 74.1%; AUC = 0.805) and bilateral mean deviation in visual CS (D) showing an overall accuracy of 73.7% (specificity = 78.3%, sensitivity = 70.6%; AUC = 0.783). Prior to evaluation for classification accuracy, bilateral mean deviation was adjusted for gender and years of education. All other variables (contrast thresholds in the bilateral mean lower left VF and bilateral mean upper right VF, exam duration) were adjusted for age at visit, gender, and years of education.
Similar articles
- Brain glutathione levels--a novel biomarker for mild cognitive impairment and Alzheimer's disease.
Mandal PK, Saharan S, Tripathi M, Murari G. Mandal PK, et al. Biol Psychiatry. 2015 Nov 15;78(10):702-10. doi: 10.1016/j.biopsych.2015.04.005. Epub 2015 Apr 14. Biol Psychiatry. 2015. PMID: 26003861 - Cognitive consequences of high Aβ amyloid in mild cognitive impairment and healthy older adults: implications for early detection of Alzheimer's disease.
Lim YY, Ellis KA, Harrington K, Kamer A, Pietrzak RH, Bush AI, Darby D, Martins RN, Masters CL, Rowe CC, Savage G, Szoeke C, Villemagne VL, Ames D, Maruff P; AIBL Research Group. Lim YY, et al. Neuropsychology. 2013 May;27(3):322-332. doi: 10.1037/a0032321. Neuropsychology. 2013. PMID: 23688214 - Visual Event-Related Potentials in Mild Cognitive Impairment and Alzheimer's Disease: A Literature Review.
Morrison C, Rabipour S, Taler V, Sheppard C, Knoefel F. Morrison C, et al. Curr Alzheimer Res. 2019;16(1):67-89. doi: 10.2174/1567205015666181022101036. Curr Alzheimer Res. 2019. PMID: 30345915 Review. - Auditory Event-related Potentials in Mild Cognitive Impairment and Alzheimer's Disease.
Morrison C, Rabipour S, Knoefel F, Sheppard C, Taler V. Morrison C, et al. Curr Alzheimer Res. 2018;15(8):702-715. doi: 10.2174/1567205015666180123123209. Curr Alzheimer Res. 2018. PMID: 29359668 Review.
Cited by
- Alzheimer's Retinopathy: Seeing Disease in the Eyes.
Mirzaei N, Shi H, Oviatt M, Doustar J, Rentsendorj A, Fuchs DT, Sheyn J, Black KL, Koronyo Y, Koronyo-Hamaoui M. Mirzaei N, et al. Front Neurosci. 2020 Sep 8;14:921. doi: 10.3389/fnins.2020.00921. eCollection 2020. Front Neurosci. 2020. PMID: 33041751 Free PMC article. Review. - Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer's disease.
Liu D, Zhang L, Li Z, Zhang X, Wu Y, Yang H, Min B, Zhang X, Ma D, Lu Y. Liu D, et al. BMC Neurol. 2015 Feb 21;15:14. doi: 10.1186/s12883-015-0268-6. BMC Neurol. 2015. PMID: 25886372 Free PMC article. - Revolution of Alzheimer Precision Neurology. Passageway of Systems Biology and Neurophysiology.
Hampel H, Toschi N, Babiloni C, Baldacci F, Black KL, Bokde ALW, Bun RS, Cacciola F, Cavedo E, Chiesa PA, Colliot O, Coman CM, Dubois B, Duggento A, Durrleman S, Ferretti MT, George N, Genthon R, Habert MO, Herholz K, Koronyo Y, Koronyo-Hamaoui M, Lamari F, Langevin T, Lehéricy S, Lorenceau J, Neri C, Nisticò R, Nyasse-Messene F, Ritchie C, Rossi S, Santarnecchi E, Sporns O, Verdooner SR, Vergallo A, Villain N, Younesi E, Garaci F, Lista S; Alzheimer Precision Medicine Initiative (APMI). Hampel H, et al. J Alzheimers Dis. 2018;64(s1):S47-S105. doi: 10.3233/JAD-179932. J Alzheimers Dis. 2018. PMID: 29562524 Free PMC article. Review. - A systematic survey of advances in retinal imaging modalities for Alzheimer's disease diagnosis.
Vij R, Arora S. Vij R, et al. Metab Brain Dis. 2022 Oct;37(7):2213-2243. doi: 10.1007/s11011-022-00927-4. Epub 2022 Mar 15. Metab Brain Dis. 2022. PMID: 35290546 Review. - A Potential Association Between Retinal Changes, Subjective Memory Impairment, and Anxiety in Older Adults at Risk for Alzheimer's Disease: A 27-Month Pilot Study.
Cheng DL, Thompson L, Snyder PJ. Cheng DL, et al. Front Aging Neurosci. 2019 Oct 29;11:288. doi: 10.3389/fnagi.2019.00288. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31736739 Free PMC article.
References
- Ahmed S, Mitchell J, Arnold R, Dawson K, Nestor PJ, Hodges JR. Memory complaints in mild cognitive impairment, worried well, and semantic dementia patients. Alzheimer Dis Assoc Disord. 2008;22(3):227–35. - PubMed
- Anderson AJ, Johnson CA. Frequency-doubling technology perimetry. Ophthalmol Clin North Am. 2003;16(2):213–25. - PubMed
- Armstrong RA. Visual field defects in Alzheimer’s disease patients may reflect differential pathology in the primary visual cortex. Optom Vis Sci. 1996;73(11):677–82. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 AG010133/AG/NIA NIH HHS/United States
- TL1 RR025759/RR/NCRR NIH HHS/United States
- RC2 AG036535/AG/NIA NIH HHS/United States
- C06-RR020128/RR/NCRR NIH HHS/United States
- P30AG10133-18S1/AG/NIA NIH HHS/United States
- R01 AG19771/AG/NIA NIH HHS/United States
- U54 RR025761/RR/NCRR NIH HHS/United States
- R01 CA101318/CA/NCI NIH HHS/United States
- R01 AG019771/AG/NIA NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- C06 RR020128/RR/NCRR NIH HHS/United States
- P30 AG10133-18S1/AG/NIA NIH HHS/United States
- U01 AG032984/AG/NIA NIH HHS/United States
- TL1RR025759/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical