Light-dependent structural change of chicken retinal Cryptochrome4 - PubMed (original) (raw)
Light-dependent structural change of chicken retinal Cryptochrome4
Ryuji Watari et al. J Biol Chem. 2012.
Abstract
Animals have several classes of cryptochromes (CRYs), some of which function as core elements of circadian clockwork, circadian photoreceptors, and/or light-dependent magnetoreceptors. In addition to the circadian clock genes Cry1 and Cry2, nonmammalian vertebrates have the Cry4 gene, the molecular function of which remains unknown. Here we analyzed chicken CRY4 (cCRY4) expression in the retina with in situ hybridization and found that cCRY4 was likely transcribed in the visual pigment cells, cells in the inner nuclear layer, and retinal ganglion cells. We further developed several monoclonal antibodies to the carboxyl-terminal extension of cCRY4 and localized cCRY4 protein with immunohistochemistry. Consistent with the results of in situ hybridization, cCRY4 immunoreactivity was found in visual pigment cells and cells located at the inner nuclear layer and the retinal ganglion cell layer. Among the antibodies, one termed C1-mAb had its epitope within the carboxyl-terminal 14-amino acid sequence (QLTRDDADDPMEMK) and associated with cCRY4 in the retinal soluble fraction more strongly in the dark than under blue light conditions. Immunoprecipitation experiments under various light conditions indicated that cCRY4 from the immunocomplex formed in the dark dissociated from C1-mAb during blue light illumination as weak as 25 μW/cm(2) and that the release occurred with not only blue but also near UV light. These results suggest that cCRY4 reversibly changes its structure within the carboxyl-terminal region in a light-dependent manner and operates as a photoreceptor or magnetoreceptor with short wavelength sensitivity in the retina.
Figures
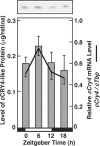
FIGURE 1.
Expression analysis of cCry transcripts and cCRY4 protein. Temporal variation of levels of cCRY4-like immunoreactivity in the retina in LD. Chick retinal homogenates were subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting with an anti-cCRY4 CCT polyclonal antibody. BlockAce was used for blocking and dilution of antibodies. A representative blotting image is shown in the upper panel. Bars in the lower panel represent levels of cCRY4-like immunoreactivity (mean ± S.E. (error bars), n = 3) estimated by comparing their signals with those of various amounts of MBP-CCT fusion proteins. Proteins equivalent to 0.01 retina were loaded in each lane. For comparison, the chick retinal cCry4 mRNA profile in LD was reproduced from Kubo et al. (10).
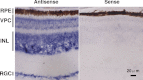
FIGURE 2.
In situ hybridization of cCry4 in the chicken retina. Left, antisense probe; right, sense-probe (control). RPE, retinal pigment epithelium.
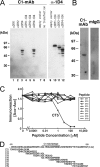
FIGURE 3.
Specificity and epitope of anti-cCRY4 C1-mAb. A, immunoblot analysis of cCRY1, cCRY2, and cCRY4 expressed in HEK293 cells. cCry1, cCry2, and cCry4 cDNAs were subcloned into the pcDNA3.1 vector. A 1D4 tag (23) was added at the C terminus of the CRYs (termed cCRY1-1D4, cCRY2-1D4, and cCRY4-1D4, respectively) to detect the expression of cCRY proteins. cCRYs with or without the 1D4 tag were overexpressed in HEK293 cells as described previously (10) and analyzed using C1- and 1D4-mAbs. B, immunoblot analysis of chick retinal proteins by C1-mAb. Whole chick retinal proteins (equivalent to 0.01 retina) were separated on 9% SDS-PAGE and visualized with immunoblotting using C1-mAb or control mouse IgG (0.1 μg/ml). BlockAce was used for the blocking and dilution of antibodies in immunoblot analysis (A and B). C, determination of the epitope region of C1-mAb using ELISA. ELISA microplate wells were coated with GST-CCT antigen, blocked with 1% skim milk, reacted with C1-mAb, and the unreacted mAb was washed out. The wells were incubated with 1% skim milk containing various concentrations of peptides (CT1-CT9, see D), followed by detection of the remaining C1-mAb through use of an HRP-labeled secondary antibody. D, sequence of the CCT region of cCRY4 and peptides used in ELISA. A tyrosine residue was added to the amino terminus of each peptide for quantitation using UV spectroscopy.
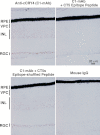
FIGURE 4.
Immunohistochemical localization of cCRY4 in the retina using C1-mAb, which recognizes CCT region of cCRY4. Mouse IgG (1 μg/ml, right panel) was used as the primary antibody instead of C1-mAb (1 μg/ml, left panel). RPE, retinal pigment epithelium.
FIGURE 5.
Immunohistochemical localization of cCRY4 in the retina and specificity of the C1-mAb. The primary antibodies (C1-mAb) were incubated overnight at 4 °C in blocking solution either with a competitor peptide (100 μ
m
; CT5 epitope peptide (YQLTRDDADDPMEMK) or CT5s epitope-shuffled peptide (YQDMRPDMDDETALK)) or without competitor (for C1-mAb and mouse IgG). RPE, retinal pigment epithelium. The signals are weaker than Fig. 4 because of the shorter incubation time for detection reaction.
FIGURE 6.
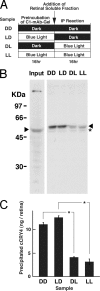
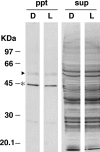
Immunoprecipitation of cCRY4 under blue light or dark conditions and the effect on precipitation efficiencies in light conditions during the preirradiation of C1-mAb-conjugated gel. A, schematic diagram of experimental procedure. B and C, cCRY4(-like immunoreactivities) eluted in the supernatants after incubation under various light conditions. C1-mAb-gel was incubated under dark (D) or blue light (L, 424 μW/cm2, 1.0 × 1015 photon/cm2/s) conditions for 16 h at 4 °C, mixed with chick retinal soluble fraction, incubated for 16 h at 4 °C under D or L conditions, and washed with wash buffer three times. The supernatants were then subjected to immunoblot analysis to estimate the relative amounts of cCRY4 protein eluted from the gel using C3 mAb (1 μg/ml), which recognizes the carboxyl-terminal CT8 region of cCRY4 (HSEESFTKTKAARM). Skim milk (1%) in TBS was used for blocking and dilution of antibodies. Proteins equivalent to those from 0.39 retina were loaded in each lane. Signals were detected using the AttoPhos fluorescent substrate (Roche Applied Science) and Typhoon 9410 scanner (GE Healthcare). A representative immunoblot image (one of the three replicates) is shown in B. Error bars represent the S.E. An asterisk in B denotes bands due to nonspecific binding of the secondary antibody. Asterisks in C denote p < 0.01.
FIGURE 7.
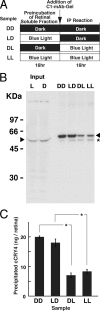
Effects on the immunoprecipitation efficiencies of cCRY4 by blue light irradiation of retinal soluble fractions during and before immunoprecipitation. A, schematic diagram of experimental procedure. B and C, cCRY4(-like immunoreactivities) eluted in the supernatants after incubation under various light conditions. Then, the chick retinal soluble fraction was divided into several aliquots, which were incubated in the dark (D) or illuminated with blue light (L, 424 μW/cm2, 1.0 × 1015 photon/cm2/s) for 16 h at 4 °C. They were mixed with the C1-mAb-gel and incubated for 16 h at 4 °C under dark (D) or blue light (L, 424 μW/cm2, 1.0 × 1015 photon/cm2/s) conditions, and the gel was washed with wash buffer three times. The supernatants were then subjected to immunoblot analysis to estimate the relative amounts of cCRY4 protein eluted from the gel using C3-mAb. Proteins equivalent to those of 0.39 retina were loaded in each lane. Skim milk (1%) in TBS was used for blocking and dilution of antibodies. Signals were detected as described in Fig. 6. A representative immunoblot image (one of the three replicates) is shown in B. An asterisk in B denotes bands due to nonspecific binding of the secondary antibody. Error bars represent the S.E. Asterisks in C denote p < 0.01.
FIGURE 8.
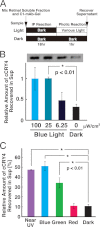
Effects on the immunoprecipitation efficiencies of cCRY4 by varying intensity and at different wavelengths of light irradiation. A, schematic diagram of experimental procedure. B, light dependence and the release of cCRY4 from the C1-mAb-gel. The chick retinal soluble fraction was mixed with C1-mAb-gel, incubated for 18 h at 4 °C, and washed with wash buffer five times. Then the gel was divided into several aliquots, which were incubated in the dark or illuminated by various intensities of blue light at 25 °C for 1 h. A representative immunoblot image (one of the three replicates) is shown in the upper part of the panel. C, wavelength dependence and the release of cCRY4 from the C1-mAb-gel. Chicken CRY4-bound gel was prepared as above, and the aliquots were incubated in the dark or irradiated with LED lights at a constant photon density (1.0 × 1015 photons/cm2 per s) but different spectral characteristics at 25 °C for 1 h. The supernatants were then subjected to immunoblot analysis using C1-mAb (0.1 μg/ml) to estimate the relative amounts of cCRY4 protein eluted from the gel. Proteins equivalent to those from 0.08 retina were loaded in each lane. Skim milk (1%) in TBS was used for blocking and dilution of antibodies. Signals were detected as described in Fig. 6. Error bars represent the S.E.
FIGURE 9.
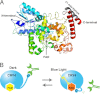
Models for light-dependent structural change cCRY4. A, model for cCRY4 and C1-mAb epitope within the carboxyl-terminal extension. A simple sequence replacement model of cCRY4 (Arg4–Glu514) with FAD chromophore was constructed based on Arabidopsis thaliana photolyase (Protein Data Bank ID code 3FY4) with the aid of MATRAS server (24) and drawn using the PyMOL Molecular Graphics System (MacPyMOL, Schrödinger, LLC). The model lacks some loop regions and amino- and carboxyl-terminal short amino acid stretches. The C1-mAb epitope within the carboxyl-terminal extension is colored in gray. B, model for the blue light-dependent interaction between cCRY4 and C1-mAb.
FIGURE 10.
Silver staining of immunoprecipitated proteins in the retinal soluble fractions. C1-mAb-gel was mixed with chick retinal soluble fraction, incubated for 7 h at 4 °C under dark or blue light (424 μW/cm2) conditions, and washed with wash buffer three times. The precipitates and supernatants were then subjected to SDS-PAGE followed by silver staining. Proteins equivalent to those from 0.05 retina were loaded in each lane. We observed no band for cCRY4 in the precipitate probably due to the low content. An arrowhead and asterisk indicate the protein bands for tubulin and β-actin, which were identified by MALDI-TOF MS (AXIMA CFR plus; Shimadzu). These two proteins showed the similar binding to a control IgG-conjugated gel, indicating their nonspecific binding to the C1-mAb-gel.
Similar articles
- Overexpression in yeast, photocycle, and in vitro structural change of an avian putative magnetoreceptor cryptochrome4.
Mitsui H, Maeda T, Yamaguchi C, Tsuji Y, Watari R, Kubo Y, Okano K, Okano T. Mitsui H, et al. Biochemistry. 2015 Mar 17;54(10):1908-17. doi: 10.1021/bi501441u. Epub 2015 Mar 4. Biochemistry. 2015. PMID: 25689419 - Molecular cloning, mRNA expression, and immunocytochemical localization of a putative blue-light photoreceptor CRY4 in the chicken pineal gland.
Kubo Y, Akiyama M, Fukada Y, Okano T. Kubo Y, et al. J Neurochem. 2006 May;97(4):1155-65. doi: 10.1111/j.1471-4159.2006.03826.x. J Neurochem. 2006. PMID: 16686694 - Expression of the blue-light receptor cryptochrome in the human retina.
Thompson CL, Bowes Rickman C, Shaw SJ, Ebright JN, Kelly U, Sancar A, Rickman DW. Thompson CL, et al. Invest Ophthalmol Vis Sci. 2003 Oct;44(10):4515-21. doi: 10.1167/iovs.03-0303. Invest Ophthalmol Vis Sci. 2003. PMID: 14507900 - The Retina and Other Light-sensitive Ocular Clocks.
Besharse JC, McMahon DG. Besharse JC, et al. J Biol Rhythms. 2016 Jun;31(3):223-43. doi: 10.1177/0748730416642657. Epub 2016 Apr 19. J Biol Rhythms. 2016. PMID: 27095816 Free PMC article. Review. - Nonvisual photoreceptors of the deep brain, pineal organs and retina.
Vigh B, Manzano MJ, Zádori A, Frank CL, Lukáts A, Röhlich P, Szél A, Dávid C. Vigh B, et al. Histol Histopathol. 2002 Apr;17(2):555-90. doi: 10.14670/HH-17.555. Histol Histopathol. 2002. PMID: 11962759 Review.
Cited by
- The biophysical, molecular, and anatomical landscape of pigeon CRY4: A candidate light-based quantal magnetosensor.
Hochstoeger T, Al Said T, Maestre D, Walter F, Vilceanu A, Pedron M, Cushion TD, Snider W, Nimpf S, Nordmann GC, Landler L, Edelman N, Kruppa L, Dürnberger G, Mechtler K, Schuechner S, Ogris E, Malkemper EP, Weber S, Schleicher E, Keays DA. Hochstoeger T, et al. Sci Adv. 2020 Aug 12;6(33):eabb9110. doi: 10.1126/sciadv.abb9110. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32851187 Free PMC article. - Chemical and structural analysis of a photoactive vertebrate cryptochrome from pigeon.
Zoltowski BD, Chelliah Y, Wickramaratne A, Jarocha L, Karki N, Xu W, Mouritsen H, Hore PJ, Hibbs RE, Green CB, Takahashi JS. Zoltowski BD, et al. Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19449-19457. doi: 10.1073/pnas.1907875116. Epub 2019 Sep 4. Proc Natl Acad Sci U S A. 2019. PMID: 31484780 Free PMC article. - Myths in magnetosensation.
Nimpf S, Keays DA. Nimpf S, et al. iScience. 2022 May 23;25(6):104454. doi: 10.1016/j.isci.2022.104454. eCollection 2022 Jun 17. iScience. 2022. PMID: 35677648 Free PMC article. Review. - Orientation of migratory birds under ultraviolet light.
Wiltschko R, Munro U, Ford H, Stapput K, Thalau P, Wiltschko W. Wiltschko R, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014 May;200(5):399-407. doi: 10.1007/s00359-014-0898-y. Epub 2014 Apr 10. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2014. PMID: 24718656 - Cryptochrome 1 in Retinal Cone Photoreceptors Suggests a Novel Functional Role in Mammals.
Nießner C, Denzau S, Malkemper EP, Gross JC, Burda H, Winklhofer M, Peichl L. Nießner C, et al. Sci Rep. 2016 Feb 22;6:21848. doi: 10.1038/srep21848. Sci Rep. 2016. PMID: 26898837 Free PMC article.
References
- Li Q. H., Yang H. Q. (2007) Cryptochrome signaling in plants. Photochem. Photobiol. 83, 94–101 - PubMed
- Oztürk N., Song S. H., Ozgür S., Selby C. P., Morrison L., Partch C., Zhong D., Sancar A. (2007) Structure and function of animal cryptochromes. Cold Spring Harb. Symp. Quant. Biol. 72, 119–131 - PubMed
- Bayram O., Braus G. H., Fischer R., Rodriguez-Romero J. (2010) Spotlight on Aspergillus nidulans photosensory systems. Fungal Genet. Biol. 47, 900–908 - PubMed
- Busza A., Emery-Le M., Rosbash M., Emery P. (2004) Roles of the two Drosophila cryptochrome structural domains in circadian photoreception. Science 304, 1503–1506 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources