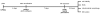Genetic risk for Parkinson's disease correlates with alterations in neuronal manganese sensitivity between two human subjects - PubMed (original) (raw)
Genetic risk for Parkinson's disease correlates with alterations in neuronal manganese sensitivity between two human subjects
Asad A Aboud et al. Neurotoxicology. 2012 Dec.
Abstract
Manganese (Mn) is an environmental risk factor for Parkinson's disease (PD). Recessive inheritance of PARK2 mutations is strongly associated with early onset PD (EOPD). It is widely assumed that the influence of PD environmental risk factors may be enhanced by the presence of PD genetic risk factors in the genetic background of individuals. However, such interactions may be difficult to predict owing to the complexities of genetic and environmental interactions. Here we examine the potential of human induced pluripotent stem (iPS) cell-derived early neural progenitor cells (NPCs) to model differences in Mn neurotoxicity between a control subject (CA) with no known PD genetic risk factors and a subject (SM) with biallelic loss-of-function mutations in PARK2 and family history of PD but no evidence of PD by neurological exam. Human iPS cells were generated from primary dermal fibroblasts of both subjects. We assessed several outcome measures associated with Mn toxicity and PD. No difference in sensitivity to Mn cytotoxicity or mitochondrial fragmentation was observed between SM and CA NPCs. However, we found that Mn exposure was associated with significantly higher reactive oxygen species (ROS) generation in SM compared to CA NPCs despite significantly less intracellular Mn accumulation. Thus, this report offers the first example of human subject-specific differences in PD-relevant environmental health related phenotypes that are consistent with pathogenic interactions between known genetic and environmental risk factors for PD.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Fig. 1. Experimental Design
iPS cells were plated for each experiment on Matrigel-covered 12 well or 96 well plates at a density of 6×104 cell/ml. On the fourth day after plating they reach a confluency of approximately 90 per cent and that is when neuralization starts (day 1) with the dual-SMAD inhibition protocol. On day 7 of neuralization 80-90% of the cell population in culture are NPCs (PAX6+). We exposed the cells in all of our experiments on day 7 for 24 hours followed by assessment of Mn-related outcome measures.
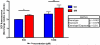
Fig. 2. Mn cytotoxicity in NPCs
CA and SM NPCs were treated with Mn and cell viability was assessed using MTT assay. Two-way ANOVA revealed significant exposure effect (p < 0.001) without a significant genotype effect (p = 0.345). CA lines used in this experiment include CA6 (n=2) and CA11 (n=2), and SM lines include SM3 (n=1), SM5 (n=1), and SM14 (n=1). Data are collected from 3 independent replicates. Error bars represent SEM.
Fig. 3. ROS generation in NPCs
NPCs are treated with two concentrations of manganese and the level of ROS generation was assessed using DCF dye. Two-way ANOVA on the log-transformed values returned genotype difference (P=0.001) but no genotype × exposure interaction. CA lines used in these experiments include CA6 (n=3) and CA11 (n=3); and SM lines include SM3 (n=2) and SM14 (n=2). Data are collected from four independent 500 μM and six independent 1000 μM MnCl2 experiments. Error bars represent SEM.
Fig. 4. Quantification of mitochondrial fragmentation in NPCs using MitoTracker Red
CA and SM NPCs are treated with 500μM MnCl2 for 24 hours followed by mitochondrial staining using MitoTracker Red dye (100 nM). 50 PAX6+ cells were counted for each condition and scored from 1 to 4 increasing with severity of mitochondrial fragmentation. The average score of cells assessed for three independent experiments after exposure to 0 or 500 μM Mn. Two-way ANOVA on the log-transformed data returned high significance for the effect of Mn exposure, but no genotype or genotype × exposure interaction effects. Paired two-tailed t-test was used for post hoc analysis of the three independent paired experiments: CA6 with SM4 (n=1), and CA6 with SM5 (n=2). Error bars represent SEM.
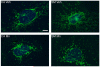
Fig. 5. Quantification of mitochondrial fragmentation in NPCs using CellLight Mitochondria-GFP
Representative images are shown for CA and SM vehicle- and Mn-treated NPCs after staining with CellLight Mitochondria-GFP and TO-PRO-3 nuclear counterstain. PAX6+ NPCs were analyzed for mitochondrial fragmentation as described in section 2.5. (CA6: SM3 n=2, CA11:SM14 n=1). Scale bar = 10 μm.
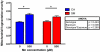
Fig. 6. Measurement of total Mn accumulation in SM and CA NPCs using (GFAAS)
Intracellular Mn accumulation was assessed in CA and SM NPCs after exposure to vehicle and 500 μM Mn. Outputs were normalized to the protein concentration of each sample. Two-way ANOVA revealed significant genotype difference in total Mn level after Mn exposure (P = 009). CA6 and SM5 lines were used in these experiments. Data are collected from 3 independent vehicle exposed samples and 4 independent Mn treated samples. Error bars represent SEM.
Similar articles
- PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper.
Aboud AA, Tidball AM, Kumar KK, Neely MD, Han B, Ess KC, Hong CC, Erikson KM, Hedera P, Bowman AB. Aboud AA, et al. Neurobiol Dis. 2015 Jan;73:204-12. doi: 10.1016/j.nbd.2014.10.002. Epub 2014 Oct 12. Neurobiol Dis. 2015. PMID: 25315681 Free PMC article. - α-Synuclein protects against manganese neurotoxic insult during the early stages of exposure in a dopaminergic cell model of Parkinson's disease.
Harischandra DS, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. Harischandra DS, et al. Toxicol Sci. 2015 Feb;143(2):454-68. doi: 10.1093/toxsci/kfu247. Epub 2014 Nov 21. Toxicol Sci. 2015. PMID: 25416158 Free PMC article. - Higher vulnerability and stress sensitivity of neuronal precursor cells carrying an alpha-synuclein gene triplication.
Flierl A, Oliveira LM, Falomir-Lockhart LJ, Mak SK, Hesley J, Soldner F, Arndt-Jovin DJ, Jaenisch R, Langston JW, Jovin TM, Schüle B. Flierl A, et al. PLoS One. 2014 Nov 12;9(11):e112413. doi: 10.1371/journal.pone.0112413. eCollection 2014. PLoS One. 2014. PMID: 25390032 Free PMC article. - Are there common biochemical and molecular mechanisms controlling manganism and parkisonism.
Roth JA. Roth JA. Neuromolecular Med. 2009;11(4):281-96. doi: 10.1007/s12017-009-8088-8. Epub 2009 Sep 16. Neuromolecular Med. 2009. PMID: 19757210 Review. - Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.
Perfeito R, Cunha-Oliveira T, Rego AC. Perfeito R, et al. Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
Cited by
- Growth and heavy metal accumulation of Koelreuteria paniculata seedlings and their potential for restoring manganese mine wastelands in Hunan, China.
Huang Z, Xiang W, Ma Y, Lei P, Tian D, Deng X, Yan W, Fang X. Huang Z, et al. Int J Environ Res Public Health. 2015 Feb 3;12(2):1726-44. doi: 10.3390/ijerph120201726. Int J Environ Res Public Health. 2015. PMID: 25654773 Free PMC article. - Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells.
Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H, Anantharam V, Huang X, Lewis M, Kanthasamy A, Kanthasamy AG. Sarkar S, et al. Sci Signal. 2019 Jan 8;12(563):eaat9900. doi: 10.1126/scisignal.aat9900. Sci Signal. 2019. PMID: 30622196 Free PMC article. - Differential responses of induced pluripotent stem cell-derived cardiomyocytes to anisotropic strain depends on disease status.
Chun YW, Voyles DE, Rath R, Hofmeister LH, Boire TC, Wilcox H, Lee JH, Bellan LM, Hong CC, Sung HJ. Chun YW, et al. J Biomech. 2015 Nov 5;48(14):3890-6. doi: 10.1016/j.jbiomech.2015.09.028. Epub 2015 Oct 8. J Biomech. 2015. PMID: 26476764 Free PMC article. - PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper.
Aboud AA, Tidball AM, Kumar KK, Neely MD, Han B, Ess KC, Hong CC, Erikson KM, Hedera P, Bowman AB. Aboud AA, et al. Neurobiol Dis. 2015 Jan;73:204-12. doi: 10.1016/j.nbd.2014.10.002. Epub 2014 Oct 12. Neurobiol Dis. 2015. PMID: 25315681 Free PMC article. - Manganese-induced Neurotoxicity: From C. elegans to Humans.
Chen P, Chakraborty S, Peres TV, Bowman AB, Aschner M. Chen P, et al. Toxicol Res (Camb). 2015 Mar 1;4(2):191-202. doi: 10.1039/C4TX00127C. Toxicol Res (Camb). 2015. PMID: 25893090 Free PMC article.
References
- Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM. Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain Res. 2009;1281:1–14. - PMC - PubMed
- Bowler RM, Gocheva V, Harris M, Ngo L, Abdelouahab N, Wilkinson J, Doty RL, Park R, Roels HA. Prospective study on neurotoxic effects in manganese-exposed bridge construction welders. Neurotoxicology. 2011;32:596–605. - PubMed
- Brown S, Taylor NL. Could mitochondrial dysfunction play a role in manganese toxicity? Environ Toxicol Pharmacol. 1999;7:49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES000267/ES/NIEHS NIH HHS/United States
- T32 GM07347/GM/NIGMS NIH HHS/United States
- P01 GM085354/GM/NIGMS NIH HHS/United States
- ES016931-02S1/ES/NIEHS NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- R01 ES016931/ES/NIEHS NIH HHS/United States
- ES016931/ES/NIEHS NIH HHS/United States
- NS078289/NS/NINDS NIH HHS/United States
- R01 NS078289/NS/NINDS NIH HHS/United States
- 5P30 ES000267/ES/NIEHS NIH HHS/United States
- 5P01 GM08535403/GM/NIGMS NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- P30HD15052/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical