Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex - PubMed (original) (raw)
Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex
Maki Hoshiko et al. J Neurosci. 2012.
Abstract
Accumulative evidence indicates that microglial cells influence the normal development of brain synapses. Yet, the mechanisms by which these immune cells target maturating synapses and influence their functional development at early postnatal stages remain poorly understood. Here, we analyzed the role of CX3CR1, a microglial receptor activated by the neuronal chemokine CX3CL1 (or fractalkine) which controls key functions of microglial cells. In the whisker-related barrel field of the mouse somatosensory cortex, we show that the recruitment of microglia to the sites where developing thalamocortical synapses are concentrated (i.e., the barrel centers) occurs only after postnatal day 5 and is controlled by the fractalkine/CX3CR1 signaling pathway. Indeed, at this developmental stage fractalkine is overexpressed within the barrels and CX3CR1 deficiency delays microglial cell recruitment into the barrel centers. Functional analysis of thalamocortical synapses shows that CX3CR1 deficiency also delays the functional maturation of postsynaptic glutamate receptors which normally occurs at these synapses between the first and second postnatal week. These results show that reciprocal interactions between neurons and microglial cells control the functional maturation of cortical synapses.
Figures
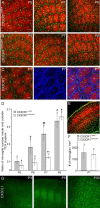
Figure 1.
CX3CL1/R1 signaling controls the entry of microglia into TCA clusters. A, B, Merge confocal images of tangential sections through layer 4 showing TCA (red, anti-5-HTT) and fluorescent microglia (green) of the barrel field of P5, P7, and P9 CX3CR1+/eGFP (A) and CX3CR1eGFP/eGFP (B) mice. C, Confocal images of a tangential section through layer 4 of a P7 CX3CR1+/+ mouse showing microglia (green, anti-Iba1), TCAs (red, anti-VGlut2) and nuclei (blue, TO-PRO-3). D, Comparison of the ratio of microglia number inside and outside TCA clusters in CX3CR1+/eGFP and CX3CR1eGFP/eGFP mice. *p < 0.02, **p < 0.01 between CX3CR1+/eGFP and CX3CR1eGFP/eGFP mice; #p < 0.05, ##p < 0.01 between P5 and other ages; Mann–Whitney U test. E, Coronal section of the barrel cortex of a P7 CX3CR1eGFP/eGFP used to count microglial cells (green) in layer 4 (red, TCAs, anti-5-HTT). F, Comparable microglia density in layer 4 of CX3CR1+/eGFP and CX3CR1eGFP/eGFP mice (p = 0.44, Mann–Whitney U test). G, Postnatal development of CX3CL1 (fractalkine) immunoreactivity in coronal sections of the mouse somatosensory cortex. Scale bars: A, E, 100 μm; C, 50 μm; G, 200 μm. Numbers above bar histograms refer to animal numbers.
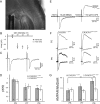
Figure 2.
CX3CR1 deficiency impairs the functional maturation of thalamocortical synapses. A, DIC image of a P7 thalamocortical slice with the recording pipette in a barrel and the bipolar stimulating electrode in the internal capsule. Scale bar, 200 μm. B, Peak amplitude of individual (black dots) and mean (white circles) thalamocortical EPSCs plotted as a function of the stimulation intensity for the determination of the minimal stimulation (22 V in this example). C, Same cell as in B, thalamocortical EPSCs evoked by two stimulations for the determination of the paired-pulse ratio. D, Similar paired-pulse ratios of thalamocortical EPSCs in CX3CR1+/eGFP and CX3CR1eGFP/eGFP mice at P5, P7, and P9. Statistical differences between P5 and other ages within each genotype are indicated. E, Effect of NBQX and
d
-AP-5 on thalamocortical EPSCs evoked in a P9 neuron. F, AMPAR- and NMDAR-mediated EPSCs in layer 4 neurons of P5 and P9 CX3CR1+/eGFP and CX3CR1eGFP/eGFP mice. G, Comparison of the AMPAR/NMDAR ratio for P5, P7, and P9 CX3CR1+/eGFP and CX3CR1eGFP/eGFP mice. Each trace is an average of 15–20 individual sweeps. *p < 0.05, **p < 0.01 (unpaired t test with Welch correction).
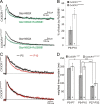
Figure 3.
CX3CR1 deficiency impairs the GluN2B to GluN2A developmental switch occurring at thalamocortical synapses between the first and second postnatal week. A, Effect of 300 n
m
Ro256981 on averaged (15–20 sweeps) NMDAR-mediated EPSCs in P10 neurons of CX3CR1+/eGFP (upper traces) and CX3CR1eGFP/eGFP (lower traces) mice. B, Summary of Ro256981 effects on NMDAR-mediated current charges for P9–P10 neurons. C, Normalized average traces of NMDAR-mediated currents recorded in layer 4 neurons of P5 (black) and P10 (red) of CX3CR1+/eGFP (upper traces) and CX3CR1eGFP/eGFP (lower traces) mice. D, Comparison of the weighted decay time constant in P5–P7, P9–P10, and adult CX3CR1+/eGFP and CX3CR1eGFP/eGFP mice. *p < 0.05, **p < 0.01,***p < 0.001 (unpaired t test with Welch correction).
Similar articles
- Adaptive phenotype of microglial cells during the normal postnatal development of the somatosensory "Barrel" cortex.
Arnoux I, Hoshiko M, Mandavy L, Avignone E, Yamamoto N, Audinat E. Arnoux I, et al. Glia. 2013 Oct;61(10):1582-94. doi: 10.1002/glia.22503. Epub 2013 Jul 26. Glia. 2013. PMID: 23893820 - CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer's disease mouse models.
Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. Lee S, et al. Am J Pathol. 2010 Nov;177(5):2549-62. doi: 10.2353/ajpath.2010.100265. Epub 2010 Sep 23. Am J Pathol. 2010. PMID: 20864679 Free PMC article. - Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide.
Corona AW, Huang Y, O'Connor JC, Dantzer R, Kelley KW, Popovich PG, Godbout JP. Corona AW, et al. J Neuroinflammation. 2010 Dec 17;7:93. doi: 10.1186/1742-2094-7-93. J Neuroinflammation. 2010. PMID: 21167054 Free PMC article. - Fractalkine Signaling and Microglia Functions in the Developing Brain.
Arnoux I, Audinat E. Arnoux I, et al. Neural Plast. 2015;2015:689404. doi: 10.1155/2015/689404. Epub 2015 Aug 4. Neural Plast. 2015. PMID: 26347402 Free PMC article. Review. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
Cited by
- Loss of P2Y12 Has Behavioral Effects in the Adult Mouse.
Lowery RL, Mendes MS, Sanders BT, Murphy AJ, Whitelaw BS, Lamantia CE, Majewska AK. Lowery RL, et al. Int J Mol Sci. 2021 Feb 13;22(4):1868. doi: 10.3390/ijms22041868. Int J Mol Sci. 2021. PMID: 33668516 Free PMC article. - LTD-like molecular pathways in developmental synaptic pruning.
Piochon C, Kano M, Hansel C. Piochon C, et al. Nat Neurosci. 2016 Sep 27;19(10):1299-310. doi: 10.1038/nn.4389. Nat Neurosci. 2016. PMID: 27669991 Free PMC article. Review. - Synaptic and extrasynaptic location of the receptor tyrosine kinase met during postnatal development in the mouse neocortex and hippocampus.
Eagleson KL, Milner TA, Xie Z, Levitt P. Eagleson KL, et al. J Comp Neurol. 2013 Oct 1;521(14):3241-59. doi: 10.1002/cne.23343. J Comp Neurol. 2013. PMID: 23787772 Free PMC article. - Perineuronal Net Alterations Following Early-Life Stress: Are Microglia Pulling Some Strings?
Rahimian R, Belliveau C, Simard S, Turecki G, Mechawar N. Rahimian R, et al. Biomolecules. 2024 Aug 30;14(9):1087. doi: 10.3390/biom14091087. Biomolecules. 2024. PMID: 39334854 Free PMC article. Review. - A Developmental Analysis of Juxtavascular Microglia Dynamics and Interactions with the Vasculature.
Mondo E, Becker SC, Kautzman AG, Schifferer M, Baer CE, Chen J, Huang EJ, Simons M, Schafer DP. Mondo E, et al. J Neurosci. 2020 Aug 19;40(34):6503-6521. doi: 10.1523/JNEUROSCI.3006-19.2020. Epub 2020 Jul 13. J Neurosci. 2020. PMID: 32661024 Free PMC article.
References
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. - PubMed
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. - PubMed
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous