Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells - PubMed (original) (raw)
. 2012 Nov 29;491(7426):769-73.
doi: 10.1038/nature11583. Epub 2012 Oct 28.
Itai M Pessach, Shen-Ying Zhang, Michael J Ciancanelli, Melina Herman, Avinash Abhyankar, Shui-Wang Ying, Sotirios Keros, Peter A Goldstein, Gustavo Mostoslavsky, Jose Ordovas-Montanes, Emmanuelle Jouanguy, Sabine Plancoulaine, Edmund Tu, Yechiel Elkabetz, Saleh Al-Muhsen, Marc Tardieu, Thorsten M Schlaeger, George Q Daley, Laurent Abel, Jean-Laurent Casanova, Lorenz Studer, Luigi D Notarangelo
Affiliations
- PMID: 23103873
- PMCID: PMC3527075
- DOI: 10.1038/nature11583
Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells
Fabien G Lafaille et al. Nature. 2012.
Abstract
In the course of primary infection with herpes simplex virus 1 (HSV-1), children with inborn errors of toll-like receptor 3 (TLR3) immunity are prone to HSV-1 encephalitis (HSE). We tested the hypothesis that the pathogenesis of HSE involves non-haematopoietic CNS-resident cells. We derived induced pluripotent stem cells (iPSCs) from the dermal fibroblasts of TLR3- and UNC-93B-deficient patients and from controls. These iPSCs were differentiated into highly purified populations of neural stem cells (NSCs), neurons, astrocytes and oligodendrocytes. The induction of interferon-β (IFN-β) and/or IFN-λ1 in response to stimulation by the dsRNA analogue polyinosinic:polycytidylic acid (poly(I:C)) was dependent on TLR3 and UNC-93B in all cells tested. However, the induction of IFN-β and IFN-λ1 in response to HSV-1 infection was impaired selectively in UNC-93B-deficient neurons and oligodendrocytes. These cells were also much more susceptible to HSV-1 infection than control cells, whereas UNC-93B-deficient NSCs and astrocytes were not. TLR3-deficient neurons were also found to be susceptible to HSV-1 infection. The rescue of UNC-93B- and TLR3-deficient cells with the corresponding wild-type allele showed that the genetic defect was the cause of the poly(I:C) and HSV-1 phenotypes. The viral infection phenotype was rescued further by treatment with exogenous IFN-α or IFN-β ( IFN-α/β) but not IFN-λ1. Thus, impaired TLR3- and UNC-93B-dependent IFN-α/β intrinsic immunity to HSV-1 in the CNS, in neurons and oligodendrocytes in particular, may underlie the pathogenesis of HSE in children with TLR3-pathway deficiencies.
Conflict of interest statement
Author Information
The authors have no competing financial interests to declare.
Figures
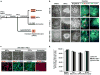
Figure 1. Derivation and purification of CNS cells
a- Schematic diagram of the differentiation and purification protocols used. b- Representative phase-contrast images showing the morphology of the hPSCs, neural rosettes and NPC clusters, from healthy control hESCs, healthy control iPSCs, and UNC-93B−/− iPSCs. Immunocytochemistry analysis revealed the expression of rosette markers (PLZF/ZO1) and a forebrain marker FOXG1. c- Characterization of UNC-93B−/− iPSC-derived CNS cell types:
Upper panels
: phase-contrast images illustrating the characteristic morphology of each type of neural cell;
Lower panels
: immunofluorescence analysis for markers of neural stem cells (nestin), neurons (Tuj1), oligodendrocyte progenitor cells (O4) and astrocytes (GFAP). **d-**Quantification of marker expression for each neural cell type derived from control hESCs, UNC-93B−/− iPSCs, and control iPSCs (error bars = SEM). * Scale bars in b- correspond to 100 μm; those in c- correspond to 50 μm, except for O4 staining (100 μm).
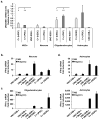
Figure 2. UNC-93B-dependent IFN responses to TLR3 in neurons and glial cells
a- UNC93B1 mRNA levels in CNS cells differentiated from hESCs from a healthy control (C+ (hESC)) and iPSCs from a healthy control (C+ (iPSC)) and an UNC-93B-deficient patient (UNC-93B−/−). b-, c- IFN-β (b-) or IFN-γ1 (c-) mRNA induction, after six hours of poly(I:C) stimulation, in neurons (b-) or oligodendrocytes (c-) differentiated from one healthy control hESC line, one healthy control iPSC line, and in UNC-93B−/− neurons (b-) or oligodendrocytes (c-), without lentiviral infection, or after infection with a lentivirus containing human WT UNC93B1 (UNC-93B−/− + UNC93B1) or a mock construct (UNC-93B−/− + mock). d- IFN-β (upper panel) or IFN-γ1 (lower panel) mRNA induction, after four (upper panel) or six (lower panel) hours of poly(I:C) stimulation, in astrocytes differentiated from hESCs from a healthy control, in UNC-93B−/− astrocytes, without lentiviral infection, or after infection with a lentivirus containing human WT UNC93B1 (UNC-93B−/− + UNC93B1) or a mock construct (UNC-93B−/− + mock). In a- to d-, Mean values ± SEM were calculated from three (a-) or two (b–d) independent experiments, each carried out in duplicate. ANOVA was performed for data shown in a-. When significant, Dunnett t tests were performed for 2X2 comparisons, significant results are indicated in corresponding panels (* p<0.05, ** p<0.01).
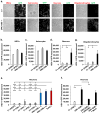
Figure 3. High HSV-1 susceptibility in UNC-93B-deficient neurons and oligodendrocytes
24 hours of infection with HSV-1-GFP, at a multiplicity of infection of 1, was performed in experiments shown. a- GFP expression in CNS cells differentiated from UNC-93B−/− iPSCs or from hESCs from a healthy control (C+) was visualized by fluorescence microscopy. Phase-contrast photomicrographs from the same view are also shown. b-, c-, d- GFP expression in NSCs (b-), astrocytes (c-) and neurons (d-) differentiated from UNC-93B−/− iPSCs, from hESCs from a healthy control (C+ (hESC)) and one to two iPSC lines each from up to three healthy controls (C_+_ (iPSC)), was assessed with a fluorescence plate reader. The difference in GFP intensity between HSV-1-GFP-infected cells and uninfected cells is shown. e- GFP expression in neurons differentiated from two lines of UNC-93B−/− iPSCs, one line of TLR3−/− iPSCs, one or two iPSC lines each from three healthy controls, or from one C+ hESCs line. f- GFP expression, in neurons from one C+ iPSC line, in UNC-93B−/− neurons, without lentiviral infection, or after infection with a lentivirus containing human WT UNC93B1 (UNC-93B−/− + UNC93B1) or a mock construct (UNC-93B−/− + Mock). g- GFP expression in oligodendrocytes differentiated from UNC-93B−/− iPSCs, from a C+ hESC line and a C+ iPSC line. Error bars = SEM, calculated from two to three experiments, from the C+ hESC, from the C+ iPSC lines and from the UNC-93B−/− lines, each studied in triplicate (b-, c-, d-, e- and g-); or from one experiment carried out in triplicate, representative of two independent experiments (f-). ANOVA was performed for the data shown in b- to g-. When significant, Dunnett t tests were performed for 2X2 comparisons, significant results are indicated in corresponding panels (* p<0.05, ** p<0.01, *** p<0.001).
Comment in
- Herpes simplex virus encephalitis: toll-free access to the brain.
Leib DA. Leib DA. Cell Host Microbe. 2012 Dec 13;12(6):731-2. doi: 10.1016/j.chom.2012.11.005. Cell Host Microbe. 2012. PMID: 23245315 Free PMC article.
Similar articles
- Human SNORA31 variations impair cortical neuron-intrinsic immunity to HSV-1 and underlie herpes simplex encephalitis.
Lafaille FG, Harschnitz O, Lee YS, Zhang P, Hasek ML, Kerner G, Itan Y, Ewaleifoh O, Rapaport F, Carlile TM, Carter-Timofte ME, Paquet D, Dobbs K, Zimmer B, Gao D, Rojas-Duran MF, Kwart D, Rattina V, Ciancanelli MJ, McAlpine JL, Lorenzo L, Boucherit S, Rozenberg F, Halwani R, Henry B, Amenzoui N, Alsum Z, Marques L, Church JA, Al-Muhsen S, Tardieu M, Bousfiha AA, Paludan SR, Mogensen TH, Quintana-Murci L, Tessier-Lavigne M, Smith GA, Notarangelo LD, Studer L, Gilbert W, Abel L, Casanova JL, Zhang SY. Lafaille FG, et al. Nat Med. 2019 Dec;25(12):1873-1884. doi: 10.1038/s41591-019-0672-3. Epub 2019 Dec 5. Nat Med. 2019. PMID: 31806906 Free PMC article. - TLR3 controls constitutive IFN-β antiviral immunity in human fibroblasts and cortical neurons.
Gao D, Ciancanelli MJ, Zhang P, Harschnitz O, Bondet V, Hasek M, Chen J, Mu X, Itan Y, Cobat A, Sancho-Shimizu V, Bigio B, Lorenzo L, Ciceri G, McAlpine J, Anguiano E, Jouanguy E, Chaussabel D, Meyts I, Diamond MS, Abel L, Hur S, Smith GA, Notarangelo L, Duffy D, Studer L, Casanova JL, Zhang SY. Gao D, et al. J Clin Invest. 2021 Jan 4;131(1):e134529. doi: 10.1172/JCI134529. J Clin Invest. 2021. PMID: 33393505 Free PMC article. - Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood.
Herman M, Ciancanelli M, Ou YH, Lorenzo L, Klaudel-Dreszler M, Pauwels E, Sancho-Shimizu V, Pérez de Diego R, Abhyankar A, Israelsson E, Guo Y, Cardon A, Rozenberg F, Lebon P, Tardieu M, Heropolitanska-Pliszka E, Chaussabel D, White MA, Abel L, Zhang SY, Casanova JL. Herman M, et al. J Exp Med. 2012 Aug 27;209(9):1567-82. doi: 10.1084/jem.20111316. Epub 2012 Jul 30. J Exp Med. 2012. PMID: 22851595 Free PMC article. - Mendelian predisposition to herpes simplex encephalitis.
Zhang SY, Abel L, Casanova JL. Zhang SY, et al. Handb Clin Neurol. 2013;112:1091-7. doi: 10.1016/B978-0-444-52910-7.00027-1. Handb Clin Neurol. 2013. PMID: 23622315 Review. - Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses.
Zhang SY, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Zhang SY, et al. Immunol Rev. 2007 Dec;220(1):225-36. doi: 10.1111/j.1600-065X.2007.00564.x. Immunol Rev. 2007. PMID: 17979850 Free PMC article. Review.
Cited by
- Diminished toll-like receptor response in febrile infection-related epilepsy syndrome (FIRES).
Hsieh MY, Lin JJ, Hsia SH, Huang JL, Yeh KW, Chang KW, Lee WI. Hsieh MY, et al. Biomed J. 2020 Jun;43(3):293-304. doi: 10.1016/j.bj.2020.05.007. Epub 2020 May 29. Biomed J. 2020. PMID: 32651134 Free PMC article. - Functional prediction of differentially expressed lncRNAs in HSV-1 infected human foreskin fibroblasts.
Hu B, Huo Y, Chen G, Yang L, Wu D, Zhou J. Hu B, et al. Virol J. 2016 Aug 5;13:137. doi: 10.1186/s12985-016-0592-5. Virol J. 2016. PMID: 27496175 Free PMC article. - Human iPSC-derived trigeminal neurons lack constitutive TLR3-dependent immunity that protects cortical neurons from HSV-1 infection.
Zimmer B, Ewaleifoh O, Harschnitz O, Lee YS, Peneau C, McAlpine JL, Liu B, Tchieu J, Steinbeck JA, Lafaille F, Volpi S, Notarangelo LD, Casanova JL, Zhang SY, Smith GA, Studer L. Zimmer B, et al. Proc Natl Acad Sci U S A. 2018 Sep 11;115(37):E8775-E8782. doi: 10.1073/pnas.1809853115. Epub 2018 Aug 28. Proc Natl Acad Sci U S A. 2018. PMID: 30154162 Free PMC article. - Global Proteomic and Methylome Analysis in Human Induced Pluripotent Stem Cells Reveals Overexpression of a Human TLR3 Affecting Proper Innate Immune Response Signaling.
Requena J, Alvarez-Palomo AB, Codina-Pascual M, Delgado-Morales R, Moran S, Esteller M, Sal M, Juan M, Boronat Barado A, Consiglio A, Bogle OA, Wolvetang E, Ovchinnikov D, Alvarez I, Jaraquemada D, Mezquita-Pla J, Oliva R, Edel MJ. Requena J, et al. Stem Cells. 2019 Apr;37(4):476-488. doi: 10.1002/stem.2966. Epub 2019 Feb 14. Stem Cells. 2019. PMID: 30664289 Free PMC article. - Intrinsic antiviral immunity of barrier cells revealed by an iPSC-derived blood-brain barrier cellular model.
Cheng Y, Medina A, Yao Z, Basu M, Natekar JP, Lang J, Sanchez E, Nkembo MB, Xu C, Qian X, Nguyen PTT, Wen Z, Song H, Ming GL, Kumar M, Brinton MA, Li MMH, Tang H. Cheng Y, et al. Cell Rep. 2022 May 31;39(9):110885. doi: 10.1016/j.celrep.2022.110885. Cell Rep. 2022. PMID: 35649379 Free PMC article.
References
- Casrouge A, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. - PubMed
- Zhang SY, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. - PubMed
- Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–148. - PubMed
- Abel L, et al. Age-dependent Mendelian predisposition to herpes simplex virus type 1 encephalitis in childhood. J Pediatr. 2010;157:623–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000043/TR/NCATS NIH HHS/United States
- R01 NS072381/NS/NINDS NIH HHS/United States
- R01 NS066390/NS/NINDS NIH HHS/United States
- 1R03AI0883502-01/AI/NIAID NIH HHS/United States
- K12 NS066274/NS/NINDS NIH HHS/United States
- 5R01NS072381-02/NS/NINDS NIH HHS/United States
- 8UL1TR000043/TR/NCATS NIH HHS/United States
- R03 AI088352/AI/NIAID NIH HHS/United States
- 1R01NS066390/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases