Building a nuclear envelope at the end of mitosis: coordinating membrane reorganization, nuclear pore complex assembly, and chromatin de-condensation - PubMed (original) (raw)
Review
Building a nuclear envelope at the end of mitosis: coordinating membrane reorganization, nuclear pore complex assembly, and chromatin de-condensation
Allana Schooley et al. Chromosoma. 2012 Dec.
Abstract
The metazoan nucleus is disassembled and re-built at every mitotic cell division. The nuclear envelope, including nuclear pore complexes, breaks down at the beginning of mitosis to accommodate the capture of massively condensed chromosomes by the spindle apparatus. At the end of mitosis, a nuclear envelope is newly formed around each set of segregating and de-condensing chromatin. We review the current understanding of the membrane restructuring events involved in the formation of the nuclear membrane sheets of the envelope, the mechanisms governing nuclear pore complex assembly and integration in the nascent nuclear membranes, and the regulated coordination of these events with chromatin de-condensation.
Figures
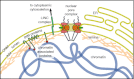
Fig. 1
The vertebrate nuclear envelope. The two-membrane sheets of the nuclear envelope are separated by a lumenal space and are continuous with the bulk endoplasmic reticulum (ER) network. The outer nuclear membrane (ONM) and the inner nuclear membrane (INM) are fused at nuclear pores, where nuclear pore complexes are integrated to regulate bidirectional transport between the cytoplasm and the nucleoplasm. The INM is distinctly characterized by a set of integral membrane proteins that connect the nuclear envelope to chromatin by interacting directly or indirectly via chromatin-associated proteins and the nuclear lamina. The nuclear lamina is additionally connected to the cytoplasmic cytoskeleton by the interaction of LINC complex proteins of the ONM and INM across the NE lumen
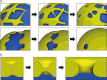
Fig. 2
The nuclear envelope is constructed by the re-organization of the mitotic ER on the chromatin. Two models have been proposed to explain nuclear envelope formation based on the predominant organization of the ER during mitosis. In the first model (a), a tubular ER network contacts chromatin via tubule ends, which flatten and expand on the chromatin surface to give rise to nuclear envelope sheets. Alternatively, ER-derived membrane sheets initiate nuclear envelope formation by associating laterally with the chromatin mass and spreading around it (b). In both cases, the regulated recruitment of membrane proteins of the INM (inset, see “Regulating the recruitment of nuclear envelope membranes to chromatin”) mediates the accumulation of nuclear envelope-specific membranes and thus the establishment of this distinct ER subdomain
Fig. 3
Membrane fusion is required for nuclear envelope formation. Cytoplasmic fusion between outgrowing ER-derived tubules (a, upper) or sheets (a, lower) is required for re-assembly of a nuclear envelope around the chromatin mass at the end of mitosis. A second type of fusion between the outer and INMs across the lumenal space is required to create a pore in the intact nuclear envelope (b) for the insertion of NPCs during interphase and possibly post-mitotically
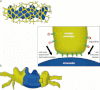
Fig. 4
Post-mitotic NPC assembly as envisioned by the insertion and enclosure models. As the cisternal sheets of the nuclear membrane wrap around chromatin, NPC assembly proceeds by either insertion into the locally intact nuclear envelope (a) or by enclosure of NPC assembly intermediates by the outgrowing membranes (b). In both cases, NPC assembly is initiated by the Mel-28/ELYS-dependent recruitment of the Nup107-160 complex to chromatin. Following the initial contact between nuclear membranes and the Nup107-160 complex, additional nucleoporins are incorporated in the assembling NPCs (see also Fig. 5 for details)
Fig. 5
Model for the ordered assembly of NPCs at the end of mitosis (see text for details and alternative models). The DNA-binding nucleoporin Mel-28/ELYS initiates NPC assembly on the chromatin by recruiting the Nup107-160 complex (a), which in turn associates with the nuclear envelope membranes via the transmembrane nucleoporin POM121 (b). The recruitment of the Nup93 complex is mediated by its membrane-associated nucleoporins, Nup53 and Nup155, which interact with integral membrane proteins at the nascent pore membrane (c) and promote the incorporation of Nup93, Nup188, and Nup205 to complete the structural backbone of the NPC (d). The subsequent recruitment of FG-repeat containing nucleoporins of the Nup62 complex (e) combined with the previous association Nup98 (not shown) establishes the central channel, a hydrophobic meshwork that confers the transport properties of the NPC. The fully assembled NPC (f) consists of multiple copies of the component nucleoporins, which are arranged in octagonal symmetry to create a cylindrical channel. Peripheral structures include the cytoplasmic filaments and the nuclear basket, protruding from opposite faces of the NPC. Initial membrane contact (b) is depicted according to the enclosure model. It should be noted that the order of events is the same for both the enclosure and insertion modes of NPC assembly
Similar articles
- Formation of the postmitotic nuclear envelope from extended ER cisternae precedes nuclear pore assembly.
Lu L, Ladinsky MS, Kirchhausen T. Lu L, et al. J Cell Biol. 2011 Aug 8;194(3):425-40. doi: 10.1083/jcb.201012063. J Cell Biol. 2011. PMID: 21825076 Free PMC article. - The Dynamic Nature of the Nuclear Envelope.
De Magistris P, Antonin W. De Magistris P, et al. Curr Biol. 2018 Apr 23;28(8):R487-R497. doi: 10.1016/j.cub.2018.01.073. Curr Biol. 2018. PMID: 29689232 Review. - Mitotic disassembly and reassembly of nuclear pore complexes.
Kutay U, Jühlen R, Antonin W. Kutay U, et al. Trends Cell Biol. 2021 Dec;31(12):1019-1033. doi: 10.1016/j.tcb.2021.06.011. Epub 2021 Jul 19. Trends Cell Biol. 2021. PMID: 34294532 Review. - Regulation and coordination of nuclear envelope and nuclear pore complex assembly.
Clever M, Mimura Y, Funakoshi T, Imamoto N. Clever M, et al. Nucleus. 2013 Mar-Apr;4(2):105-14. doi: 10.4161/nucl.23796. Epub 2013 Feb 14. Nucleus. 2013. PMID: 23412657 Free PMC article. - Nuclear pore biogenesis into an intact nuclear envelope.
Doucet CM, Hetzer MW. Doucet CM, et al. Chromosoma. 2010 Oct;119(5):469-77. doi: 10.1007/s00412-010-0289-2. Epub 2010 Aug 19. Chromosoma. 2010. PMID: 20721671 Review.
Cited by
- Sizing and shaping the nucleus: mechanisms and significance.
Jevtić P, Edens LJ, Vuković LD, Levy DL. Jevtić P, et al. Curr Opin Cell Biol. 2014 Jun;28:16-27. doi: 10.1016/j.ceb.2014.01.003. Epub 2014 Feb 4. Curr Opin Cell Biol. 2014. PMID: 24503411 Free PMC article. Review. - The mitotic protein NuMA plays a spindle-independent role in nuclear formation and mechanics.
Serra-Marques A, Houtekamer R, Hintzen D, Canty JT, Yildiz A, Dumont S. Serra-Marques A, et al. J Cell Biol. 2020 Dec 7;219(12):e202004202. doi: 10.1083/jcb.202004202. J Cell Biol. 2020. PMID: 33044554 Free PMC article. - Global Phosphoproteomic Mapping of Early Mitotic Exit in Human Cells Identifies Novel Substrate Dephosphorylation Motifs.
McCloy RA, Parker BL, Rogers S, Chaudhuri R, Gayevskiy V, Hoffman NJ, Ali N, Watkins DN, Daly RJ, James DE, Lorca T, Castro A, Burgess A. McCloy RA, et al. Mol Cell Proteomics. 2015 Aug;14(8):2194-212. doi: 10.1074/mcp.M114.046938. Epub 2015 Jun 8. Mol Cell Proteomics. 2015. PMID: 26055452 Free PMC article. - Postmitotic expansion of cell nuclei requires nuclear actin filament bundling by α-actinin 4.
Krippner S, Winkelmeier J, Knerr J, Brandt DT, Virant D, Schwan C, Endesfelder U, Grosse R. Krippner S, et al. EMBO Rep. 2020 Nov 5;21(11):e50758. doi: 10.15252/embr.202050758. Epub 2020 Sep 22. EMBO Rep. 2020. PMID: 32959960 Free PMC article. - An amphipathic helix in Brl1 is required for nuclear pore complex biogenesis in S. cerevisiae.
Kralt A, Wojtynek M, Fischer JS, Agote-Aran A, Mancini R, Dultz E, Noor E, Uliana F, Tatarek-Nossol M, Antonin W, Onischenko E, Medalia O, Weis K. Kralt A, et al. Elife. 2022 Aug 24;11:e78385. doi: 10.7554/eLife.78385. Elife. 2022. PMID: 36000978 Free PMC article.
References
- Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nat Rev Genet. 2007;8(7):507–517. - PubMed
- Anderson DJ, Hetzer MW. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol. 2007;9(10):1160–1166. - PubMed
- Anderson DJ, Hetzer MW. Shaping the endoplasmic reticulum into the nuclear envelope. J Cell Sci. 2008;121(Pt 2):137–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources